The incidence of anal cancer has been increasing over the last 30 years in the United States as well as globally.2,3 This is likely related to the increase in infections by the sexually transmitted HPV and HIV, which may have a significant impact on anal cancer incidence. In one large case-control series, an increasing number of sexual partners was associated with the development of anal cancer in both men and women (odds ratio of 4.5 for women and 2.5 for men with ≥10 sexual partners). This study also demonstrated that a history of anal warts was associated with a higher risk of developing anal cancer, as was receptive anal intercourse in women.3
Human Papillomavirus Infection
High-risk HPV type-16 has been detected in almost 90% of cases of squamous cell carcinoma of the anus.4 A recent meta-analysis suggests that HPV-16 is found more frequently (75%) and HPV-18 less frequently (10%) in anal carcinomas than in cervical carcinomas. Moreover, approximately 80% of anal cancers demonstrated more than one HPV genotype.5 Anal cancer, now considered to be a predominantly HPV-related cancer, has an incidence 15 times higher in homosexual men than in heterosexual men.6 In most cases, anal infection with HPV is sexually transmitted, and the risk for cancer is increased in patients with a history of receptive anal intercourse in women and homosexual activity in men.7 It is also been shown that women with high-grade cervical or vulvar dysplasia are more susceptible to develop anal cancer, as cervical or vulvar HPV infection escalates anal HPV infection risk.8
HIV Infection
The incidence of anal cancer in patients who are infected with HIV is estimated to be twice that of HIV-negative patients. Highly active antiretroviral therapy (HAART) has resulted in patients with HIV living longer and the development of related malignancies. In contrast to other HIV-associated malignancies, the incidence of anal cancer has actually risen following implementation of HAART.9–11 According to the National Cancer Institute (NCI), the rise in anal cancer incidence rates during 1980 to 2005 was predominantly seen in male patients with HIV, relative to their female counterparts.12 Although HIV has been considered to be a major factor in anal cancer incidence, it is also suggested that HIV may have an impact on the survival of patients with anal cancer, with one report demonstrating that HIV-positive patients with anal cancer tended to develop earlier recurrences than HIV-negative patients by 20 months, although the median survival for HIV-positive patients (34 months) and HIV-negative patients (39 months) were similar (non-significant) (discussed as follows).13
Other Risk Factors
According to the American Society of Colon and Rectal Surgeons (ASCRS), risk factors other than HIV and HPV infections include:
■ Age: 67% >55 years.
■ Smoking: There are reports demonstrating that that smoking is a risk factor for anal cancer development. According to one study, the relationship between smoking and anal cancer persists for both gender types (adjusted odds ratio for women = 3.8, 95% confidence interval [CI], 2.3 to 6.2; adjusted odds ratio for men = 3.9, 95% CI, 1.9 to 8.0). Similarly, the risk of anal cancer appears to be related to the pack-year history of smoking, with more extensive histories associated with a higher risk.14
■ Immunosuppression: Solid organ transplant recipients with chronic immunosuppressive therapy have a six times higher risk to develop anal cancer relative to the general population.15
Benign anal lesions are no longer thought to contribute to the development of this disease, although anal cancers are frequently misdiagnosed as these conditions.
Anal squamous intraepithelial lesions (SIL) or anal intraepithelial neoplasia (AIN) have been recognized as precursors that can progress to anal squamous cell carcinoma (SCC).16 Although there are no published guidelines that recommend screening of the general population for anal cancer, there are high-risk groups that may benefit from such, most prominently patients infected with HIV. Although the natural history of SIL is still being unraveled, studies show increased rates of progression in HIV-positive patients with a relative risk of 2.4 and increasing to 3.1 for those with CD4 counts below 200.17,18 The rationale for screening for SIL is based on the following: there is a high incidence of the anal cancer within the proposed screening population (i.e., HIV-positive patients), available screening tests are effective and cost-efficient, and early detection can change the outcome of the disease. The initial recommended screening test is an “anal Pap smear” that evaluates cells in the anal canal for abnormal cytology through swabbing. Patients with abnormal cytology should then be evaluated by high-resolution anoscopy, which facilitates the visualization of abnormal lesions, allowing biopsy and/or removal.19,20 Algorithms for the management of low-grade and high-grade SILs remain controversial as more data are needed to determine the effectiveness of intervention on decreasing long-term rates of anal SCC.
Treatment options of high-grade AIN include ablation with electrocautery, topical trichloroacetic acid, topical 5-FU, or imiquimod,21–23 with estimated lesion control rates ranging from 60% to 80%. A 2012 Cochrane Review highlights the dearth of evidence addressing the efficacy of available interventions for SIL. This review resulted in one randomized trial being identified. This trial evaluated the medication imiquimod versus placebo. Although underpowered, results showed no statistically significant benefit for treatment. The authors concluded that, given the rising incidence of AIN and anal cancer, well-designed randomized controlled trials are urgently needed to address this topic, with emphasis on AIN resolution, downstaging, recurrence, and progression to invasive disease in both HIV-positive and -negative populations.24
A promising strategy for the prevention of anal dysplasia and malignancy is HPV vaccination. Two vaccines (Cervarix and Gardasil) are now approved by the U.S. Food and Drug Administration and have been shown to protect against cervical cancer in women.25,26 The quadrivalent HPV vaccine Gardasil has demonstrated efficacy for prevention of HPV 6-, 11-, 16-, and 18-related genital warts and has been shown to protect against cancers of the anus, vagina, and vulva.27 In a large, double blind study, 602 healthy men who have sex with men were randomized to receive the quadrivalent HPV vaccine versus placebo. With a 36-month median follow-up for the development of AIN and/or high-risk HPV infection, significantly reduced rates of high-grade anal dysplasia and high-risk HPV infection were demonstrated in the vaccinated group.28 No cases of anal cancer or vaccine-related serious events were noted. In the context of limited availability and suboptimal outcomes of AIN screening programs, vaccination may reflect the best long-term approach for reducing anal cancer risk and is recommended for girls and boys at age 11 or 12 years and girls 13 to 26 years of age who have not been previously vaccinated.
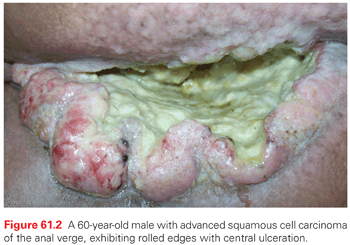
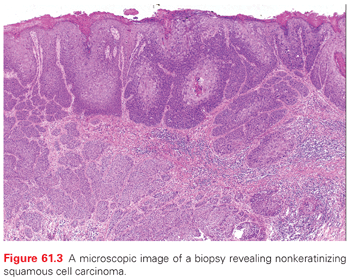
A variety of malignancies can arise in the anal canal and perianal skin. The typical gross appearance of SCC of the anal canal consists of a lesion with rolled edges, often with central ulceration, with a minority consisting of polypoid lesions (Fig. 61.2). On a practical level, these can be divided into squamous and nonsquamous histologies. The vast majority of anal canal tumors are classified as SCC, which encompasses tumors previously described as basaloid, cloacogenic, transitional, mucoepidermoid, and verrucous mucoepidermoid varieties (Fig. 61.3). These subtypes generally referred to tumors arising in the anal transitional zone, where the anal squamous histology transitions into the glandular epithelium seen in the colorectum. From a treatment standpoint, these are all approached as SCC. The current World Health Organization (WHO)29 classification does not include these subtypes. The majority of these are nonkeratinizing, although tumors arising below the dentate line often display keratinizing properties. Most squamous lesions are moderately to poorly differentiated and display koilocytic changes consistent with HPV infection.
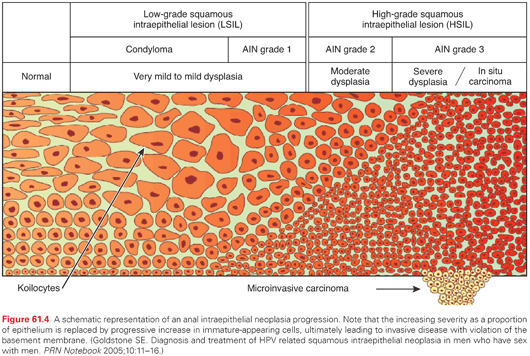
It is believed that most anal cancers arise from precancerous changes (i.e., AIN) of the anal canal and perianal skin epithelium. AIN is a multifocal process associated with HPV, analogous to cervical dysplasia. There is a progression from normal epithelium to condyloma and grade I AIN (associated with mild dysplasia), later progressing to grade II AIN (with moderate dysplasia), and ultimately grade III AIN with severe dysplasia, as well as in situ disease. Once disease has reached grade III AIN, it rarely regresses. It has been estimated that approximately 5% of AIN III patients progress to invasive malignancy, often occurring over a multiyear period. This incidence of progression of AIN III is substantially increased in patients who are immunocompromised.17,30 The prevalence of AIN among HIV-negative homosexual men is high (>36%), and almost universal among HIV-positive men who have sexual intercourse with men.31 The incidence of AIN is believed to be much greater in patients with HIV as exemplified by a French study analyzing 8,153 routine hemorrhoidectomy specimens, finding that only 3 cases of AIN (0.04%) were seen,32 as compared to 20 cases out of a 103 (19.4%) in specimens from HIV-positive men (Fig. 61.4).33
Additional nonsquamous cell histologies arising in the anal canal include adenocarcinoma, small cell carcinoma/neuroendocrine tumors, as well as undifferentiated carcinomas, melanomas, and rarely, lymphomas and sarcomas. Neuroendocrine tumors are thought to arise from endocrine cells in the transitional zone and, like neuroendocrine tumors arising from other sites, tend to disseminate widely. A suspected anal adenocarcinoma may actually reflect an extension from a distal rectal adenocarcinoma in some situations. Mucinous adenocarcinoma is generally thought to arise in the anal glands and ducts and is uncommon. It should be noted that histology is generally more important than location within the anal canal and usually dictates overall patient management.
Tumors of the perianal skin are similar to that seen in the anal canal, primarily comprised of SCC. These tumors are generally well differentiated and keratinizing. Verrucous carcinoma—also sometimes known as a giant condyloma or Buschke-Lowenstein tumor—was initially described in 1925. These tumors are sometimes mistakenly considered to be benign and misdiagnosed as condylomata acuminata, with a subsequent histologic analysis revealing invasion. These are often locally destructive and HPV related. They are often slow growing and can be present for many years before coming to medical attention. Local recurrence rates following excision are high and malignant transformation can be seen in up to 50%.34 Additional precursor lesions seen in the perianal skin include Bowen disease, which consist of a slow growing intraepidermal SCC that may mimic perianal dermatitis, as well as Paget disease, which is similar to the entity seen associated with breast cancer, with an eczematous appearance. Approximately half of Paget disease patients will harbor an underlying adenocarcinoma, notably in the colorectum.
CLINICAL PRESENTATION AND STAGING
Symptoms of anal cancer can be diverse and include bleeding, pain, sensation of a mass, itching, anal discharge, tenesmus, and a sense of fullness or a lump in the anal canal. The most common presentation is bleeding from the anus. More extensive lesions may present with more ominous symptoms such as incontinence, passage of gas or stool from the vagina, or significant change in bowel habits. Less frequently, an enlarged inguinal lymph node is reported, and 20% of patients are initially asymptomatic. Symptoms may often be dismissed as hemorrhoids or other benign causes, and it is crucial to further evaluate by a physical examination and anoscopy. Any mass should be biopsied for a diagnosis.
Clinical staging is performed by a combination of clinical, endoscopic, and radiographic examinations (Table 61.1). History should include an assessment of anal sphincter function as well as HIV risk factors. Digital rectal examination can identify fixation to the sphincter complex or adjacent organs such as the vagina and prostate. Proctoscopy provides information about the extent of mucosal spread, including the relationship to the dentate line, and facilitates biopsy. It may be necessary to examine patients under anesthesia secondary to pain and sphincter muscle spasms. Female patients should undergo a gynecologic examination to determine vaginal involvement and to exclude other HPV-associated cancers, including evaluation of the cervix. Imaging is used to better delineate the local extent of disease and regional adenopathy, and to determine the presence of distant metastases. An endoanal ultrasound or pelvic magnetic resonance imaging (MRI) can be considered to assess tumor size and involvement with local structures such as the anal sphincters and vagina or prostate. Computed tomography (CT) scans of the chest, abdomen, and pelvis are commonly employed to evaluate distant disease and adenopathy, particularly in the inguinal regions. Of all patients presenting with palpable inguinal lymph nodes, only 50% are malignant; therefore, fine-needle aspiration is often recommended in suspected cases, and a positive result may guide radiation field design and dose. Some oncologists have suggested a routine sentinel lymph node (SLN) evaluation as a staging technique. A systematic review of 16 published series evaluating the outcome of SLN biopsy of inguinal nodes included 323 patients, and the success in identifying the SLN was 86%. However, the exact role of SNL in the pretreatment evaluation remains undetermined.35
A number of recent studies have investigated the role of positron-emission tomography (PET)-CT scans for staging anal cancer. One review described outcomes of patients undergoing conventional staging with ultrasound as well as PET-CT scans. Of 95 patients, the authors found that PET-CT scans were particularly valuable in detecting more extensive nodal and metastatic disease as well as the detection of synchronous malignancies. Upstaging was noted in 14% of patients, and 23% had a change in treatment plan relative to ultrasound staging.36 Another study comparing the use of conventional imaging with CT scans and MRI found that the addition of PET-CT scans upstaged patients in 20%, downstaged 25%, and altered management in 37%. These authors concluded that PET-CT scans should be a routine part of initial staging of all anal cancers. A lesser impact was seen in patients evaluated in follow-up where PET-CT scans upstaged 11% and downstaged in 6% of cases; however, these changes also led to an altered management in 17%, indicating a potential role for selected PET-CT scan use when there is a question of recurrence or salvage surgery is planned.37 A study by Vercellino and colleagues38 similarly found that PET-CT scans were useful in the avoidance of unnecessary biopsies and surgery, with a negative predictive value of 94%, ultimately impacting on treatment plans in 22% of follow-up cases. Finally, PET-CT scans may have prognostic value as well, with one study demonstrating a significant correlation between metabolic response posttreatment and progression-free as well as overall survival.39 National Comprehensive Cancer Network (NCCN) treatment guidelines include the use of FDG-PET scans with CT scans as part of the staging evaluation, notably for patients with T2-4N0 disease or those with involved lymph nodes.40 After the previously mentioned evaluation, staging is assigned according to the American Joint Committee on Cancer 2010 (AJCC) (Table 61.2).
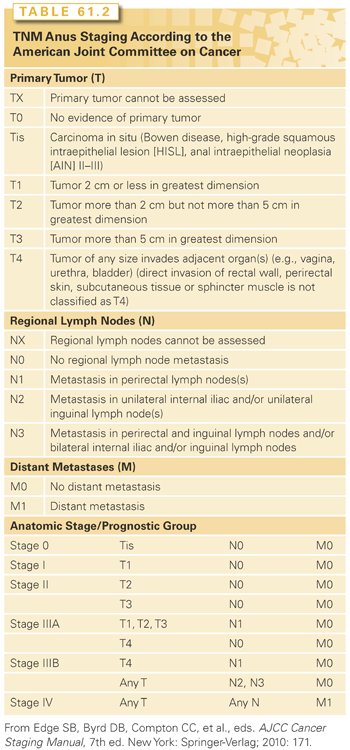
Clinical
T and N stages are the most important prognostic factors for anal cancer. According to the AJCC, the 5-year observed overall survival (OS) rates of anal cancer for stage I, II, IIIA, IIIB, and IV are 69.5%, 68.1%, 45.6%, 39.6%, and 15.3%, respectively. It has also been demonstrated that the 5-year OS rates for T1, T2, T3, T4, N0, and node-positive anal cancer are 86%, 86%, 60%, 45%, 76%, and 54%.41
There are relatively few studies available that analyze prognostic factors for anal cancer. According to the Radiation Therapy Oncology Group (RTOG) 98-11 study, tumor size (>5 cm), involved lymph nodes (N+), and male sex were associated with worse 5-year disease-free survival (DFS) and OS.42 Results from European Organisation for Research and Treatment of Cancer (EORTC) 22861 study indicated that skin ulceration, lymph node involvement, and male sex were independent variables associated with local–regional failure (LRF) and OS in multivariate analysis.43 A secondary analysis of the UK Coordinating Committee on Cancer Research (CCCR) Anal Cancer Trial (ACT) 1 trial indicated that palpable lymph nodes as well as male sex portended a poor prognosis, similar to previous studies. In addition, after adjusting for these factors, these investigators also reported that lower hemoglobin and higher white blood cell counts were also prognostic in terms of anal cancer death and worsened overall survival, respectively. Tumor site, T classification, and platelet levels had no influence on outcomes.44 However, not all studies have confirmed gender as an independent prognostic factor in terms of local control, metastasis, or overall survival.45,46
Although well-differentiated histologies have been shown to portend more favorable outcomes in other cancers, the histologic subtypes of anal cancer have not yet been demonstrated as substantial prognostic factors.47 According to a study by Schlienger and colleagues,48 there was no significant difference found in survival analysis among cloacogenic, well-differentiated, and moderately or poorly differentiated anal carcinomas.
Molecular
Prognostic biomarkers provide information on patient outcomes regardless of therapy, whereas predictive biomarkers provide information about the effect of specific therapeutic intervention.49 The ultimate goal of determining prognostic factors is to improve patient survival. Disease processes and progression result from molecular and pathologic pathways, and by targeting altered pathways, potential avenues for therapeutic interventions that could facilitate improvements in survival may emerge. Similarly, molecular analyses of anal cancer to predict treatment response and survival outcomes are essential. Lampejo and colleagues50 performed a systematic review on anal cancer prognostic biomarkers after reviewing 29 studies for final analysis. The authors found that 13 biomarkers were associated with anal cancer outcomes in only one study, whereas the tumor suppressor genes p53 and p21 as prognostic markers were established by more than one study.
The p53 gene is located in the short arm of chromosome 17 (17p13.1), which encodes a protein (393-amino acid nuclear phosphoprotein) that regulates the cell cycle and is responsible for cell apoptosis.51,52 Accumulation of nuclear proteins was seen by immunohistochemistry in the cases of muted p53 genes in some studies.53–55 These studies found that in anal carcinoma, p53 was overexpressed with a range of 34% to 100%. Wong et al.52 described that increased p53 expression was associated with worse local–regional control (p = 0.02) and DFS (p = 0.01). Another study of 64 patients suggested that mutant p53 was responsible for inferior LRF rates relative to wild type, although nonsignificant (48 versus 27%, p = 0.14).56 Allal and colleagues57 reported that anal cancer patients with p53-positive lesions had a lower local–regional control rate (relative risk [RR], 0.38; p = 0.03) and shorter DFS (RR, 0.29; p = 0.003).
The p21 gene protein, a cyclin-dependent kinase (CDK) inhibitor, is considered to be a mediator of the p53 gene function.58 Some studies have indicated that the lack of p21 expression is associated with poor prognosis in patients with squamous cell carcinoma of the anal canal (SCCA). Holm et al.59 reported that a lack of p21 expression was associated with reduced OS (p = 0.013), whereas Nilsson and colleagues60 reported that absence of the same p21 expression was also responsible for an increased LRF rate (p < 0.05).
Ajani and colleagues55 reported epidermal growth factor receptor (EGFR) expression was observed in 86% of patients with anal cancer, but the study was unable to determine the significant correlation between degree of staining and DFS. The same study’s multivariate models suggested that Ki67 (negatively, coefficient: −0.04), nuclear factor kappa B (NF-κB) (positively, coefficient: 0.07), Sonic Hedgehog (SHH) (positively, coefficient: 0.05), and Gli-1 (positively, coefficient: 0.03) were associated with DFS (p = 0.005, 0.002, 0.02, and 0.02, respectively). Further investigation into the molecular prognostic factors of anal carcinoma is needed.50
TREATMENT OF LOCALIZED SQUAMOUS CELL CARCINOMA
Surgery
As with any disease, treatment decisions in anal cancer are made following a consideration of the risks and benefits. Wide local excision may be considered in a highly select subset of patients of anal cancer patients. This approach should generally be reserved for small, well-differentiated, superficial lesions confined to the anal margin, not involving the internal sphincter, and without nodal involvement.61 For lesions that have residual microscopic disease or have close surgical margins less than 1 mm, further local excision, if technically possible, may be pursued, or adjuvant chemoradiation should be considered.40
Prior to the mid 1970s, the standard surgical approach to anal cancer was abdominal perineal resection (APR), which required a permanent colostomy following the removal of the rectum, ischiorectal fat, levator sling, perirectal and superior hemorrhoidal nodes, as well as a wide area of perianal skin, with associated long-term sequelae of sexual and urinary dysfunction. Collectively, long-term DFS results with a surgery alone approach were approximately 50%. A review at the Mayo Clinic of 118 anal cancers treated with APR reported an OS rate of 70% and overall recurrence rate of 40%. Of these, greater than 80% established recurrence sites had either local recurrence alone or some component of such.62 Similarly, other surgical series using surgery alone results suggested high rates of regional recurrence (up to 60%) following local excision or APR alone across a variety of stages.63
Definitive Chemoradiotherapy
Given the relatively poor outcomes obtained with APR alone, Nigro et al., from Wayne State University pioneered incorporating concurrent pelvic radiation therapy and chemotherapy (5-FU and MMC) prior to surgical resection, resulting in high rates of pathologic complete response and survival, later verified by other investigators.64–69 This led to significant interest in a definitive chemoradiotherapy approach.
Initially, the UK CCCR conducted the ACT I study. In this study, 585 patients with SCC of the anal canal or perianal skin were randomized to receive radiation therapy alone (45 Gy in 20 or 25 fractions), followed by additional radiation dose in patients achieving at least a 50% response (delivering an additional 15 to 25 Gy using either external beam radiation therapy or brachytherapy following a 6-week break) versus a similar radiation approach delivered concurrently with infusional 5-FU (750 mg/m2 or 1,000 mg/m2 for 5 days given during the first and last weeks of radiation therapy) along with MMC (12 mg/m2 delivered on day 1 of treatment). Note that ACT I local failure rates captured several events, including persistence of primary disease following combined modality therapy, the requirement for surgery or colostomy because of treatment failure, as well as ongoing requirement for colostomy at 6 months following the completion of treatment. On the initial report, following a median follow-up of 42 months, the 3-year local failure rate was significantly lower in patients receiving chemoradiotherapy (39% versus 61%; p <0.0001), although at the expense of increased treatment-related morbidity. In particular, hematologic, skin, GI, and genitourinary toxicities were higher with the addition of chemotherapy, with six (2%) treatment-related deaths in the chemoradiation arm versus two (0.7%) for the radiation-alone arm. Although no statistically significant advantage was seen in terms of OS (3-year OS 72% versus 65%; p = 0.17), with the addition of chemotherapy, anal cancer-related mortality was significantly improved (28% versus 39%; p = 0.02), leading the authors to conclude that patients with SCC of the anal canal could be treated with definitive chemoradiotherapy with surgery reserved for salvage. In a report of long-term follow-up of this trial, no significant differences were seen in terms of long-term morbidity, although an excess in non–anal cancer deaths was seen in the combined modality group in the first 10 years following treatment (cardiovascular, treatment related, pulmonary disease, and second malignancy), but not beyond. LRF rates remained significantly higher in patients treated with radiation therapy alone (25% absolute difference at 12 years), with a corresponding improvement in relapse-free survival (12% absolute at 12 years) through the addition of chemotherapy, with no significant difference reported between the two groups in terms of late morbidity.70,71 Although not statistically significant, an absolute improvement in 12-year survival of 5.6% was seen.
A similar but smaller trial conducted by the EORTC randomized 103 patients with T3-4N0-3 or T1-2N1-3 anal cancer to radiation therapy (45 Gy, with a boost dose of 15 to 20 Gy following a 6-week break, based on disease response), with or without concurrent chemotherapy using infusional 5-FU (750 mg/m2 per day, days 1 through 5 and days 29 through 33) with MMC (given on day 1 at 15 mg/m2). Surgery was reserved for patients with less than a partial response. Outcomes from this study revealed that patients treated with concurrent chemotherapy had a higher complete response rate (80% versus 54%) with significant improvement in local–regional control (68 versus 50%), colostomy-free (72% versus 40%) and event-free survival with the addition of chemotherapy at 5 years. Similarly, OS was improved in patients receiving chemotherapy (3 year: 72% versus 65%), although this did not reach statistical significance. Rates of high-grade toxicity were deemed similar between the two groups, although rates of late anal ulceration were higher with the use of concurrent chemotherapy.43
The results from these two trials demonstrated that the addition of chemotherapy to definitive radiation therapy significantly improves LRF rates as well as relapse-free and colostomy-free survival rates, although at the expense of increased toxicity.
The Role of Mitomycin in the Combined Modality Therapy Approach of Anal Cancer
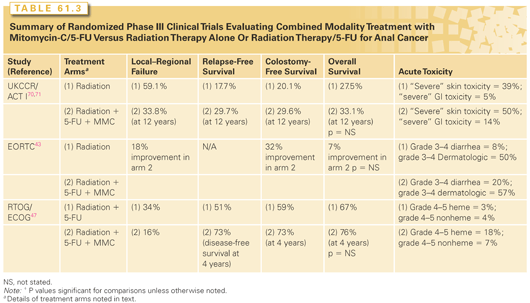
Although MMC was delivered concurrently with 5-FU in the aforementioned trials, it’s necessity was questioned given the association with significant toxicities, including significant myelosuppression, dermatitis, as well as less common side effects of pulmonary fibrosis, hemolytic-uremic syndrome and therapy-related myelodysplastic syndrome. Given this, a randomized trial conducted by the RTOG and ECOG attempted to deintensify therapy by omitting MMC in efforts to preserve oncologic efficacy while eliminating MMC-related toxicity. In this study, patients with anal cancer of any T or N stage were randomized to radiation therapy (45 to 50.4 Gy, with an additional 9 Gy to patients with biopsy-proven persistence of disease 4 to 6 weeks following the completion of initial therapy) with infusional 5-FU (1,000 mg/m2 per day on days 1 to 4 and days 29 to 32), with patients randomized to MMC (10 mg/m2 days 1 and 29) versus not. Note that in patients with a positive biopsy following the first 4- to 6-week treatment, an additional cycle of cisplatin and 5-FU with radiotherapy was delivered. A second biopsy was later obtained and, if positive for residual tumor, patients proceeded to APR. This study enrolled 310 patients, of which 291 were analyzable. Colostomy-free and disease-free survival rates were significantly higher in patients receiving MMC (71% versus 59% and 73% versus 51%, respectively) with significantly lower colostomy (9% versus 22%) and local failure rates (16% versus 34%). OS was not improved, although as in the previously described randomized studies, was numerically superior (76% versus 67%). Also of note is that all grade 4 and 5 toxicities were considerably higher in patients receiving MMC.47 Table 61.3 summarizes the results of randomized phase III clinical trials evaluating combined modality therapy with radiation, 5-FU, and mitomycin versus radiation therapy alone or radiation therapy with 5-FU.
The Role of Cisplatin Concurrent with Radiation Therapy in Anal Cancer
Given the aforementioned toxicities associated with MMC, there has been interest in substituting with a platinum-based chemotherapy when delivered concurrently with radiation therapy. Initial results using this approach from a Cancer and Leukemia Group B (CALGB) pilot study demonstrated that the use of induction 5-FU with cisplatin for advanced anal cancers (T3 to 4, or node positive) followed by definitive chemoradiotherapy (45 Gy, with or without a 9 Gy boost) with 5-FU and MMC resulted in an 80% complete response rates with 56% and colostomy-free survival.72 These and other data led to increasing interest in substituting cisplatin for MMC. The RTOG subsequently conducted a phase III trial (RTOG 98-11), which randomized 644 patients to (1) a standard arm of concurrent chemoradiotherapy (45 to 59 Gy) with continuous infusional 5-FU (1,000 mg/m2 per day) and bolus MMC (10 mg/m2) weeks 1 and 5 versus (2) an experimental arm of two cycles of induction continuous 5-FU (1,000 mg/m2 per day) and bolus cisplatin (75 mg/m2) alone on weeks 1 and 5, followed by concurrent chemoradiotherapy with two cycles of continuous 5-FU (1,000 mg/m2 per day) and bolus cisplatin (75 mg/m2) on weeks 9 and 13. The trial primary endpoint was DFS and secondary endpoint included toxicity analysis. On the initial report, no significant difference was seen in 5-year DFS (60% versus 54%; p = 0.17), OS (75% versus 70%; p = 0.10), or local–regional relapse (25% versus 33%; p = 0.07). However, the 5-year colostomy rate was 10% in the mitomycin group versus 19% in the cisplatin-containing arm (p = 0.02).73 An update of this trial demonstrated a significant improvement in 5-year DFS (68% versus 58%; p = 0.005), OS (78% versus 71%; p = 0.02), and borderline colostomy-free survival (72% versus 65%; p = 0.05) in the MMC-containing arm, albeit at the expense of increased grade 3 and higher hematologic toxicities (61% versus 42%; p < 0.001). However, nonhematologic toxicity rates were equivalent in both arms, with similar rates of late toxicity.74 Critics of this study have pointed out that the prolonged the overall treatment duration associated with the use of induction chemotherapy with cisplatin may have allowed for accelerated tumoral repopulation prior to radiation therapy initiation.
A follow-up study from the UK CCCR (the ACT II trial) performed a more direct comparison of the use of cisplatin in conjunction with radiation therapy compared to MMC. In this largest of anal cancer randomized trials, 950 patients with anal or perianal skin cancer were randomized using a 2 × 2 factorial design, with patients randomized to receive infusional 5-FU (1,000 mg/m2 per day, on days 1 to 4 and days 29 to 32) with pelvic radiation therapy (50.4 Gy) plus MMC (12 mg/m2 on day 1) versus cisplatin (60 mg/m2 on day 1 and 29) with the same 5-FU–radiation regimen. A second randomization was performed following completion of the combined treatment course to no further therapy versus two additional cycles of cisplatin and 5-FU. Grade 3 to 4 acute nonhematologic toxicity rates were similar between the regimens (60% versus 65%; p = 0.17). Not unexpectantly, grade 3 and higher hematologic toxicity rates were greater in the mitomycin group (25% versus 13%; p < 0.001), although no toxic deaths were reported in the trial. In terms of the trial primary endpoint of complete clinical response at 6 months, there was no significant difference between the two groups, and at a median follow-up of 5.1 years, clinical complete response at 26 weeks was 91% in the MMC group compared to 90% in the cisplatin group. Additionally, the 3-year colostomy rate was similar between the two groups (14% mitomycin versus 11% cisplatin). The use of maintenance therapy in this trial showed no obvious benefit, with 3-year recurrence-free survival of 75% in both arms, similar OS rates between the maintenance versus no maintenance groups (85% versus 84%), with 3-year PFS rates of 74% in the maintenance group versus 73% in the nonmaintenance group. The authors concluded that MMC given concurrently with 5-FU and radiation therapy should remain the standard in the treatment of anal cancer, given the following: (1) the high grade hematologic toxicity seen with MMC did not significantly increase sepsis rates; (2) the MMC course was delivered over approximately 10 minutes compared to two courses of either all day or overnight intravenous (IV) hydration with cisplatin, with similar efficacy and overall toxicities between the regimens; (3) fewer chemotherapy cycles were required; (4) there was requirement for fewer nonchemotherapy drugs; (5) there was lesser expense; and (6) there was no risk of neuropathy.75
Another phase III trial conducted by the French Federation Nationale des Centres de Lutte Contre La Cancer ACCORD (the ACCORD-03 trial) randomized patients with stage II to III anal cancer to one of four treatment arms: (1) neoadjuvant 5-FU + cisplatin alone followed by 5-FU cisplatin radiation therapy (45 Gy) with a radiation therapy boost (15 Gy) using either external beam or brachytherapy techniques; (2) same as the first arm, except utilizing a higher dose radiation boost (20 to 25 Gy); (3) same as the first arm, but no neoadjuvant chemotherapy; and (4) same as the second arm, but no neoadjuvant chemotherapy. Of note is that a 3-week break was mandated following the completion of the initial 45 Gy, prior to boost treatments. Following a median 50-month follow-up, there was no significant difference in 5-year colostomy-free survival rates (70% to 82%). Similarly, no significant differences were seen between the arms in terms of LRF, cause-specific survival, and OS. The most intensified treatment arm of induction chemotherapy with high-dose radiation boost demonstrated a numerically improved local control (88% versus 72% to 83% on the additional arms). The trial authors indicated that induction chemotherapy does not improve outcomes, with the role of radiation dose escalation in this disease remaining uncertain, but felt that that the combination of induction chemotherapy and radiation dose escalation should be explored further.76 The results of randomized trials evaluating cisplatin are shown in Table 61.4.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








