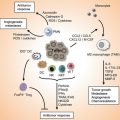
Disease
DFTD
CTVT
Species affected
Tasmanian devil
Dog, wolf, coyote, jackals, foxes
Distribution
Tasmania
Worldwide
Age of origin
About 18 years ago
At least 6,000 years ago
Likely cell of origin
Schwann cell
Macrophage
Site of primary infection
Face, mouth or neck
External genitalia
Mode of transmission
Biting
Coitus
Frequency of metastases
65 %
7 %
Mortality
100 %
Rare
Effect on host population
Decline of host population of 80 %, extinction in wild likely within 30 years
Little or no effect
Treatment
Surgical excision if treated early
Chemotherapy
22.2 Canine Transmissible Venereal Tumor
22.2.1 Prevalence and Transmission
Canine transmissible venereal tumor is a contagious neoplasm found in domestic dogs [1]. The disease is found worldwide, but is mostly prevalent in stray dog populations [2]. While it is most commonly found in dogs, it can be transmitted to a wide range of canine species including wolves, foxes, jackals and coyotes [3]. In some regions, such as Japan, it is the most common tumor found in dogs [4]. Transmission occurs by transplantation of viable tumor cells during coitus [3]. The tumor establishes on the external genital mucosa of the infected dog and can affect both sexes of any breed of dog [3]. Metastases are rare and are most commonly found in the lymph nodes [2]. CTVT in domestic dogs can be successfully treated with chemotherapy [3]. CTVT can also be induced in adult immunocompetent dogs by inoculation with living tumor cells [5].
22.2.2 Histology and Clonality
Histologically, CTVT is described as an undifferentiated round-cell neoplasm of histiocytic origin [6]. Cytologically, CTVT cells do not have many distinctive ultrastructural features [7]. CTVT has been proposed to be of macrophage lineage based on its expression of several macrophage characteristic proteins [8] and its ability to be parasitised by Leishmania infantum [9], a parasite usually infecting macrophages. The transmissibility of CTVT cells has been demonstrated by studies which found that the disease can be induced by transplanting live cells, but not killed cells or cell filtrates [3]. The chromosome number of CTVT (57–59 chromosomes) is consistent across geographically dispersed samples and is different to the normal number of dogs (76 chromosomes) [10–12]. CTVT genomes share chromosomal duplications and deletions which are not found in the dog genome [13]. Transmissibility has been further supported by the presence of a LINE insertion near the c-myc gene which is found in all CTVT tumor studied to date but is not found in the normal dog genome [14]. Recently clonal transmission of CTVT has been confirmed by molecular genetic studies. Rebbeck et al. [13] and Murgia et al. [15] found that the pattern of microsatellite polymorphism strongly suggests a monophyletic origin. The most recent common origin of CTVT may have been relatively recent, predicted to be between 47 and 470 years [13] or between 250 and 2,500 years [15]. However, the date of CTVT origin is ancient, predicted to have occurred at least 6,000 years ago in either the dog or wolf and may have predated the domestication of dogs [13]. This makes CTVT the oldest known malignant cell line.
22.2.3 Disease Progression
In experimental transplantation, the disease has a predictable growth pattern featuring three distinct stages of progressive growth, stable growth and then regression [3]. The initial progressive phase lasts several weeks and is characterised by rapid tumor growth with a doubling time of about 4–7 days [16]. During the stable growth phase, tumor expansion slows [16]. During regression, the tumor shrinks and eventually disappears [16]. Spontaneous regression is associated with an intense local lymphocytic infiltration [17]. The number and size of tumor-infiltrating lymphocyte subpopulations vary with CTVT growth phase [17]. In natural transmission, the disease will usually regress after 6–9 months of growth, unless treated earlier [2]. Experimental transplantation to immunosuppressed dogs results in tumors that do not regress [18]. Additionally, the host is immune to subsequent reinfection after remission, and offspring of infected mothers are partially protected from infection [19]. These findings demonstrate that host immune response is involved in tumor regression and protection from subsequent reinfection.
22.2.4 Immunology
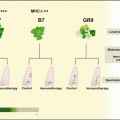
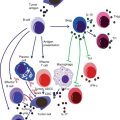
CTVT is initially capable of downregulating host immune response, but in the majority of cases, it is eventually overcome by the host defences [2]. Regression of CTVT involves both humoral and cell-mediated immunity. Rejection of foreign tissue is initiated by the presence of major histocompatibility complex (MHC) antigens (Ags) on the surface of foreign cells. MHC Ags present peptides to T cells. There are two classes of MHC antigen. Class I peptides are recognised by CD8+ T cells, while class II peptides are recognised by CD4+ T cells. Cells without MHC, mutated or foreign MHC, or MHC presenting abnormal peptides can trigger an immune response; therefore, regulation of MHC is important for cancers to escape host immunosurveillance. CTVT has the additional distinction in that it is capable of transmitting across MHC barriers. Many tumors have selective mechanisms for downregulating MHC class I molecules to escape recognition by CD8+ cells [20]. CTVT cells express none, or very few, MHC class I and II molecules during the progressive phase [21, 22]. Additionally they do not express β2M, a component of MHC class I, on the cell surface [23].
The initial lack of cell surface MHC should result in cell destruction by natural killer (NK) cells [24]. However, migration of NK cells to the tumor is impaired due to tumor expression of TGF-β1 [25]. TGF-β1 is a potent immunosuppressive cytokine which commonly plays a role in immune avoidance in cancers [26]. TGF-β1 is expressed in high concentrations in CTVT tumors where it suppresses the killing activity of tumor-infiltrating lymphocytes (TIL) [25]. Natural killer cells, which migrate to the tumor due to the lack of cell surface MHC expression, are impaired by TGF-β1 [25]. In addition, the function of dendritic cells (DCs) is impaired with inhibited antigen uptake and presentation, impaired differentiation and apoptosis of monocytes and DCs [27].
Host expression of interleukin-6 (IL-6) appears to be critical in forcing the tumor into regression. At the onset of regression, expression of IL-6 by TILs is increased, antagonising the activity of tumor TGF-β1 [25]. By downregulating TGF-β1, the ability of interferon γ (IFN-γ) to promote MHC class I and II expressions is restored [28]. IFN-γ and IL-6 work synergistically to enhance MHC expression [28]. It has been postulated that IFN-γ induces expression of an MHC class II transactivator, resulting in an increased MHC class II expression [28]. This results in the attraction of CD4+ cells which promote the generation of antibodies (Abs) against CTVT, driving tumor rejection and the subsequent immunity against it. Host IL-6 may also enhance T cell cytotoxcity when MHC molecules are expressed [25]. Additionally, during regression TILs secrete a heat-sensitive factor, enhancing MHC class I and II expressions [29]. At commencement of regression, 30–40 % of cells express both class I and II MHCs [21, 22]. DC activity is substantially recovered during regression [27]. Expression of IL-6 and the re-establishment of DCs are believed to be the critical factors in initiating tumor regression.
There is evidence that humoral immunity is also involved in regression. Treating CTVT-infected dogs with the sera of post-regressive dogs caused regression, while dogs simultaneously given CTVT and immune serum did not develop the disease [18]. Antibodies to CTVT have been found in dogs after CTVT regression [30]. B lymphocytes and plasma cells appear in higher concentration in regressive than progressive tumors [17]. Additionally, Liao et al. [31] detected a CTVT-secreted factor which was specifically cytotoxic to B cells.
22.3 Devil Facial Tumor Disease
22.3.1 Prevalence and Appearance
Tasmanian devils are the world’s largest marsupial carnivore since the extinction of the Tasmanian tiger in the early twentieth century. Devils were once widespread on mainland Australia, but today are restricted to the island of Tasmania. Although several population crashes have been reported over the last two centuries, Tasmanian devils were classified as a species of least concern prior to the outbreak of DFTD with a population of around 150,000 animals. DFTD was first witnessed in 1996 by a wildlife photographer in Mount William National Park in the far north-east of Tasmania [32]. Since then, the disease has spread rapidly across the state, with the disease found in 85 % of the devil distribution as of 2012 [33]. The disease is projected to have spread to the entirety of devil distribution by 2016 [34]. Since its emergence, DFTD has wiped out over 80 % of the devil population [34], and unless acted upon, the devil is expected to be extinct within 30 years [33]. This had led to devils being listed as endangered by the IUCN as well as national and state authorities [35].
DFTD appears as tumors mostly around the head and neck of the devil [36]. After appearance of the first lesions, death usually occurs within 6 months [36], and there have been no verified cases of devils having survived the disease. Death may occur due to starvation or complications from metastases [37]. The tumors are undifferentiated soft tissue neoplasm, believed to be derived from Schwann cell originator cells [38]. Metastases occur in around 65 % of cases [36].
22.3.2 Transmission
DFTD was discovered to be a transmissible allograft from karyotypes of the tumors and hosts [39]. Similar to CTVT, DFTD samples have a conserved karyotype which is distinct from the normal devil karyotype [39]. The DFTD karyotype is highly rearranged with the absence of both copies of chromosome 1, one copy of chromosome 5 and both sex chromosomes [39, 40]. Clonality has been confirmed by genotyping which has shown that DFTD specimens taken from different individuals are identical to each other, but usually different to their hosts, at several microsatellite markers as well as MHC genes [41]. Further support for clonality comes from next-generation sequencing [42]. The disease is spread by biting. Devils bite one another frequently when fighting over food and territory or during mating [43]. DFTD cells are transferred when a devil bites into the tumor of a diseased devil [44]. DFTD cells can then establish into a tumor around wounds in the mouth or face of the new host [39, 45].
22.3.3 Immunology
While much is known about the immunology and pathology of CTVT, very little is known about the same aspects of DFTD. Unlike CTVT, DFTD cells pass from one animal to another without provoking an immune response [46]. Both tumor and host characteristics have been hypothesised to be responsible for the ability of this cancer to spread and go undetected by the host immune system. It has been suggested that an impaired immune system and a lack of genetic diversity, in particular at MHC genes, may make the devil population susceptible to the spread of DFTD [47]. However, it is likely that the tumor itself actively avoids immune detection. Both downregulation of cell surface MHC and the expression of immunosuppressive factors have been investigated. Each of these host and tumor characteristics will be discussed below.
22.3.4 Do Devils Have an Impaired Immune System?
Soon after the emergence of DFTD, it was hypothesised that the spread of this disease may be enabled by an impaired devil immune system [47]. Tasmanian devils are known to be highly susceptible to neoplasms [48]. However, research over the last decade has demonstrated that devils have a robust immune system functionally equivalent to other marsupial and eutherian immune systems. Their immune tissue architecture and immune cell distribution are similar to that seen in eutherians; their lymphocytes proliferate in response to mitogen stimulation, and subcutaneous injection of a cellular Ag produces a strong antibody response [46, 49]. Additionally, NK-cell responses have been demonstrated [50]. Therefore, with a robust immune response, it is unlikely that the absence of immune response to DFTD is due to a lack of functionality in the devils’ immune system.
22.3.5 Devils Have Low MHC Diversity
Devils lack genetic diversity across the genome [42, 51] as well as at MHC class I and class II genes [41, 52]. Having an important role in the recognition of both cancerous and foreign cells, MHC class I presentation should be critical in the recognition of DFTD cells by the devil’s immune system. However, this recognition may be impaired if MHC diversity is so low that it impairs the host immune system from recognising these cells as foreign. Siddle et al. [41] found that devils have critically low MHC diversity and suggested that this was the first link between a lack of MHC diversity and the spread of disease. However, the role of MHC diversity in DFTD spread has been questioned by recent studies. Kriess et al. [53] conducted skin grafts in devils and found that even MHC similar hosts were capable of rejection. This suggests that minor histocompatibility Ags may play a role in allograft rejection. Most recently Lane et al. [54] found no link between MHC diversity and susceptibility to DFTD, suggesting that MHC is not critical to the disease spread. However, the lack of genetic diversity in devils may still be responsible for the spread of this disease. There is likely to be low diversity at other key immune genes such as minor histocompatibility Ags and genes of the innate immune system, which may have a role in disease susceptibility.
22.3.6 Expression of Immunosuppressive Cytokines
Expression of immunosuppressive cytokines is found in many cancers and allows the cancers to avoid host detection and destruction of tumor cells by suppressing the host immune response. As discussed, the expression of TGFβ1 by CTVT cells is involved in preventing NK-cell response [25]. However, it has been recently shown that TGFβ1 as well as three other cytokines commonly expressed by cancers to downregulate immune detection are not over-expressed in DTFD tumors compared to control tissues [55]. This includes VEGF-A, IL-6 and IL-10. It therefore appears that in DFTD, unlike CTVT, suppression of the immune system by release of immunosuppressive cytokines does not play a key role in the pathology of DFTD [56].
22.3.7 Regulation of Cell Surface MHC
Like CTVT, regulation of cell surface MHC may allow DFTD to avoid rejection. Recent research has shown that DFTD cells express functional MHC class I and class IIB RNA transcripts, but little or no transcripts for genes involved in Ag processing including B2M, TAP, MHC class IIA and DMB [56]. This study found only trace amounts of MHC I proteins at the surface of DFTD cells both in vivo and in vitro [56]. These findings may explain how DFTD cells evade recognition by T cells, though further work is needed to build a full picture of how DFTD cells avoid immune recognition.
22.4 Comparison of DFTD and CTVT
DFTD and CTVT are the only naturally occurring clonally transmissible cancers. Over the last decade, our understanding of the pathology and immunology of these diseases has greatly developed, but much is still unknown. Further research into their origin, evolution and immunology will not only provide insights into transmissible cancers, but may also have medical applications to human cancers. These two diseases differ in many aspects of their pathology and immunology. However, they also share features in common which may help reveal circumstances favoring the generation of such diseases.
While CTVT is an ancient disease having been around for at least 6000 years, DFTD is a very new disease less than 20 years old. This allows us to compare a transmissible cancer which has been through thousands of years of co-evolution with its host species to one which has recently emerged. CTVT, as a successful parasite, has likely undergone selection to become a benign tumor which does not kill its host population, resulting in the low rate of metastases and the characteristic regression. On the other hand, the newly emerged DFTD results in 100 % mortality and may drive its host population to extinction. Immunologically there are both similarities and differences in how these diseases avoid host immune rejection. CTVT initially has low or no expression of MHC classes I and II to avoid rejection by T cells, and the expression of host TGFβ1 appears to be important in preventing NK-mediated destruction of tumor cells [25]. Similarly, recent evidence has shown that DFTD has low or no expression of MHC class I on the cell surface [57]. However, TGFβ1 is not upregulated in DFTD cells [55]. Therefore, it is yet to be seen how DFTD cells avoid NK-mediated destruction.
With only two naturally occurring transmissible cancers worldwide, it is somewhat surprising that these diseases do not occur more frequently. There are several commonalities between CTVT and DFTD which appear to be significant factors making their host species susceptible to this form of disease. Firstly, for such a disease to occur, a route of transmission must be present. In both species, transmission of cancer cells appears to occur at the site of tissue damage. Devils’ biting behavior [43] and the extended, rough copulation that occurs in dogs [57] provide routes of transmission for the transfer of tumor cells. The probable low genetic diversity in both of the original host populations is another commonality. Tasmanian devils have low genetic diversity, particularly at MHC genes [41, 51], while it has been hypothesised the CTVT arose in an inbred wolf population due to homozygosity at a number of loci in CTVT [15]. Additionally, a spontaneously arising sarcoma was found to be transmissible among a colony of laboratory Syrian hamsters which also had low MHC diversity [58]. A further trait which may predispose populations to disease is susceptibility to neoplasms. Devils are naturally highly susceptible to neoplasms [48]; however, this is not a trait shared by dogs or wolves. If the presence of all these factors is indeed required for the development of transmissible cancers, this may explain the rarity of such diseases. However, with wildlife species increasingly losing diversity due to anthropogenic effects, the chance of seeing similar disease occurring in wild vertebrate species may be increasing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree