Cancer Genetic Counseling
Ellen T. Matloff
Danielle C. Bonadies
In the past 15 years, clinically based genetic testing has evolved from an uncommon analysis ordered for the rare hereditary cancer family to a widely available tool ordered on a routine basis to assist in surgical decision making, chemoprevention, and surveillance of the patient with cancer, as well as management of the entire family. The evolution of this field has created a need for accurate cancer genetic counseling and risk assessment. Extensive coverage of this topic by the media and widespread advertising by commercial testing laboratories have further fueled the demand for counseling and testing.
Cancer genetic counseling is a communication process between a health care professional and an individual concerning cancer occurrence and risk in his or her family.1 The process, which may include the entire family through a blend of genetic, medical, and psychosocial assessment and intervention, has been described as a bridge between the fields of traditional oncology and genetic counseling.1
The goals of this process include providing the client with an assessment of individual cancer risk, while offering the emotional support needed to understand and cope with this information. It also involves deciphering whether the cancers in a family are likely to be caused by a mutation in a cancer gene and, if so, which one. There are >30 hereditary cancer syndromes, many of which can be caused by mutations in different genes. Therefore, testing for these syndromes can be complicated. Advertisements by genetic testing companies bill genetic testing as a simple process that can be carried out by health care professionals with no training in this area; however, there are many genes involved in cancer, the interpretation of the test results is often complicated, the risk of result misinterpretation is great and associated with potential liability, and the emotional and psychological ramifications for the patient and family can be powerful. A few hours of training by a company generating a profit from the sale of these tests does not adequately prepare providers to offer their own genetic counseling and testing services.2 Furthermore, the delegation of genetic testing responsibilities to office staff is alarming3 and likely presents a huge liability for these ordering physicians, their practices, and their institutions. Providers should proceed with caution before taking on the role of primary genetic counselor for their patients.
Counseling about hereditary cancers differs from “traditional” genetic counseling in several ways. Clients seeking cancer genetic counseling are rarely concerned with reproductive decisions that are often the primary focus in traditional genetic counseling, but are instead seeking information about their own and other relatives’ chances of developing cancer.1 In addition, the risks given are not absolute but change over time as the family and personal history changes and the patient ages. The risk reduction options available are often radical (e.g., chemoprevention or prophylactic surgery), and are not appropriate for every patient at every age. The surveillance and management plan must be tailored to the patient’s age, childbearing status, menopausal status, risk category, ease of screening, and personal preferences, and will likely change over time with the patient. The ultimate goal of cancer genetic counseling is to help the patient reach the decision best suited to her personal situation, needs, and circumstances.
There are now a significant number of referral centers across the country specializing in cancer genetic counseling and the numbers are growing. However, some experts insist that the only way to keep up with the overwhelming demand for counseling will be to educate more physicians and nurses in cancer genetics. The feasibility of adding another specialized and time-consuming task to the clinical burden of these professionals is questionable, particularly with average patient encounters of 19.5 and 21.6 minutes for general practitioners and gynecologists, respectively.4,5 A more practical goal may be to better educate primary care providers in the area of generalized risk assessment so that they can screen their patient populations for individuals at
high risk for hereditary cancer and refer them on to comprehensive counseling and testing programs. Access to genetic counseling is no longer an issue because there are now internet-, phone-, and satellite-based telemedicine services available (Table 1.1), and several major health insurance companies now cover these services.6,7,8
high risk for hereditary cancer and refer them on to comprehensive counseling and testing programs. Access to genetic counseling is no longer an issue because there are now internet-, phone-, and satellite-based telemedicine services available (Table 1.1), and several major health insurance companies now cover these services.6,7,8
Table 1.1 How to Find a Genetic Counselor for your Patient | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Who is a Candidate for Cancer Genetic Counseling?
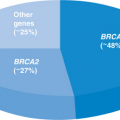
Only 5% to 10% of most cancers are due to single mutations within autosomal dominant inherited cancer susceptibility genes.9 The key for clinicians is to determine which patients are at greatest risk to carry a hereditary mutation. There are seven critical risk factors in hereditary cancer (Table 1.2). The first is early age of cancer onset. This risk factor, even in the absence of a family history, has been shown to be associated with an increased frequency of germline mutations in many types of cancers.10 The second risk factor is the presence of the same cancer in multiple affected relatives on the same side of the pedigree. These cancers do not need to be of similar histologic type in order to be caused by a single mutation. The third risk factor is the clustering of cancers known to be caused by a single gene mutation in one family (e.g., breast/ovarian/pancreatic cancer or colon/ovarian/uterine cancers). The fourth risk factor is
the occurrence of multiple primary cancers in one individual. This includes multiple primary breast or colon cancers as well as a single individual with separate cancers known to be caused by a single gene mutation (e.g., breast and ovarian cancer in a single individual). Ethnicity also plays a role in determining who is at greatest risk to carry a hereditary cancer mutation. Individuals of Jewish ancestry are at increased risk to carry three specific BRCA1/2 mutations.11 The presence of a cancer that presents unusually, in this case breast cancer in a male, represents a sixth risk factor and is important even when it is the only risk factor present. Finally, the last risk factor is pathology. This risk factor is listed in Table 1.1 in italics because it is a new and evolving entity. It appears that certain types of cancer are overrepresented in hereditary cancer families. For example, medullary breast cancer appears to be overrepresented in BRCA1 families.12 Triple-negative breast cancers (ER-, PR-, Her2-) are also overrepresented in BRCA1 families,13 and the National Comprehensive Cancer Network (NCCN) has recently updated their BRCA testing guidelines to include individuals diagnosed with a triple-negative breast cancer <age 60.14 However, breast cancer patients without these pathologic findings are not necessarily at lower risk to carry a mutation. In contrast, patients with a borderline or mucinous ovarian carcinoma appear to be at lower risk to carry a BRCA1 or BRCA2 mutation15 and may instead carry a mutation in a different gene. It is already well established that medullary thyroid carcinoma (MTC), sebaceous adenoma or carcinoma, adrenocortical carcinoma before the age of 25, and multiple adenomatous, hamartomatous, or juvenile colon polyps are indicative of other rare hereditary cancer syndromes.16,17 These risk factors should be viewed in the context of the entire family history, and must be weighed in proportion to the number of individuals who have not developed cancer. Risk assessment is often limited in families that are small or have few female relatives; in such families, a single risk factor may carry more weight.
the occurrence of multiple primary cancers in one individual. This includes multiple primary breast or colon cancers as well as a single individual with separate cancers known to be caused by a single gene mutation (e.g., breast and ovarian cancer in a single individual). Ethnicity also plays a role in determining who is at greatest risk to carry a hereditary cancer mutation. Individuals of Jewish ancestry are at increased risk to carry three specific BRCA1/2 mutations.11 The presence of a cancer that presents unusually, in this case breast cancer in a male, represents a sixth risk factor and is important even when it is the only risk factor present. Finally, the last risk factor is pathology. This risk factor is listed in Table 1.1 in italics because it is a new and evolving entity. It appears that certain types of cancer are overrepresented in hereditary cancer families. For example, medullary breast cancer appears to be overrepresented in BRCA1 families.12 Triple-negative breast cancers (ER-, PR-, Her2-) are also overrepresented in BRCA1 families,13 and the National Comprehensive Cancer Network (NCCN) has recently updated their BRCA testing guidelines to include individuals diagnosed with a triple-negative breast cancer <age 60.14 However, breast cancer patients without these pathologic findings are not necessarily at lower risk to carry a mutation. In contrast, patients with a borderline or mucinous ovarian carcinoma appear to be at lower risk to carry a BRCA1 or BRCA2 mutation15 and may instead carry a mutation in a different gene. It is already well established that medullary thyroid carcinoma (MTC), sebaceous adenoma or carcinoma, adrenocortical carcinoma before the age of 25, and multiple adenomatous, hamartomatous, or juvenile colon polyps are indicative of other rare hereditary cancer syndromes.16,17 These risk factors should be viewed in the context of the entire family history, and must be weighed in proportion to the number of individuals who have not developed cancer. Risk assessment is often limited in families that are small or have few female relatives; in such families, a single risk factor may carry more weight.
Table 1.2 Risk Factors that Warrant Genetic Counseling for Hereditary Cancer Syndromes | ||
|---|---|---|
|
A less common, but extremely important, finding is the presence of unusual physical findings or birth defects that are known to be associated with rare hereditary cancer syndromes. Examples include benign skin findings, autism, large head circumference18,19 and thyroid disorders in Cowden syndrome, ontogenic keratocysts in Gorlin syndrome,20 and desmoid tumors or dental abnormalities in familial adenomatous polyposis (FAP).21 These and other findings should prompt further investigation of the patient’s family history and consideration of a referral to genetic counseling.
In this chapter, the breast/ovarian cancer counseling session with a female patient will serve as a paradigm by which all other sessions may follow broadly. However, as testing evolves from targeted testing of 1 or 2 genes to multigene panels, genetic counseling and test interpretation will become more complex.
Components of the Cancer Genetic Counseling Session
Precounseling Information
Before coming in for genetic counseling, the counselee should be given some basic information about the process. This information, which can be imparted by telephone
or in the form of written material, should outline what the counselee can expect at each session, and what information he/she should collect before the first visit. The counselee can then begin to collect medical and family history information and pathology reports that will be essential for the genetic counseling session.
or in the form of written material, should outline what the counselee can expect at each session, and what information he/she should collect before the first visit. The counselee can then begin to collect medical and family history information and pathology reports that will be essential for the genetic counseling session.
Family History
An accurate family history is undoubtedly one of the most essential components of the cancer genetic counseling session. Optimally, a family history should include at least three generations; however, patients do not always have this information. For each individual affected with cancer, it is important to document the exact diagnosis, age at diagnosis, treatment strategies, and environmental exposures (i.e., occupational exposures, cigarettes, other agents).21 The current age of the individual, laterality, and occurrence of any other cancers must also be documented. Cancer diagnoses should be confirmed with pathology reports whenever possible. A study by Love et al.22 revealed that individuals accurately reported the primary site of cancer only 83% of the time in their first-degree relatives with cancer, and 67% and 60% of the time in second- and third-degree relatives, respectively. It is common for patients to report a uterine cancer as an ovarian cancer, or a colon polyp as an invasive colorectal cancer. These differences, although seemingly subtle to the patient, can make a tremendous difference in risk assessment. Individuals should be asked if there are any consanguineous (inbred) relationships in the family, if any relatives were born with birth defects or mental retardation, and whether other genetic diseases run in the family (e.g., Fanconi Anemia or Cowden syndrome), as these pieces of information could prove important in reaching a diagnosis.
The most common misconception in family history taking is that somehow a maternal family history of breast, ovarian, or uterine cancer is more significant than a paternal history. Conversely, many still believe that a paternal history of prostate cancer is more significant than a maternal history. Few cancer genes discovered thus far are located on the sex chromosomes, and therefore both maternal and paternal history are significant and must be explored thoroughly. It has also become necessary to elicit the spouse’s personal and family history of cancer. This has bearing on the cancer status of common children, but may also determine if children are at increased risk for a serious recessive genetic disease such as Fanconi anemia.23 Children who inherit two copies of a BRCA2 mutation (one from each parent) are now known to have this serious disorder characterized by defective DNA repair and high rates of birth defects, aplastic anemia, leukemia, and solid tumors.23 Patients should be encouraged to report changes in their family history over time (e.g., new cancer diagnoses, genetic testing results in relatives), as this may change their risk assessment and counseling.
A detailed family history should also include genetic diseases, birth defects, mental retardation, multiple miscarriages, and infant deaths. A history of certain recessive genetic diseases (e.g., ataxia telangiectasia, Fanconi anemia) can indicate that healthy family members who carry just one copy of the genetic mutation may be at increased risk to develop cancer.23,24 Other genetic disorders, such as hereditary hemorrhagic
telangiectasia, can be associated with a hereditary cancer syndrome caused by a mutation in the same gene; in this case juvenile polyposis.25
telangiectasia, can be associated with a hereditary cancer syndrome caused by a mutation in the same gene; in this case juvenile polyposis.25
Dysmorphology Screening
Congenital anomalies, benign tumors, and unusual dermatologic features occur in a large number of hereditary cancer predisposition syndromes. Examples include osteomas of the jaw in FAP, palmar pits in Gorlin syndrome, and papillomas of the lips and mucous membranes in Cowden syndrome. Obtaining an accurate past medical history of benign lesions and birth defects, and screening for such dysmorphology can greatly impact diagnosis, counseling, and testing. For example, BRCA1/2 testing is inappropriate in a patient with breast cancer who has a family history of thyroid cancer and the orocutaneous manifestations of Cowden syndrome.
Risk Assessment
Risk assessment is one of the most complicated components of the genetic counseling session. It is crucial to remember that risk assessment changes over time as the person ages and as the health status of their family members change. Risk assessment can be broken down into three separate components:
What is the chance that the counselee will develop the cancer observed in his/her family (or a genetically related cancer such as ovarian cancer due to a family history of breast cancer)?
What is the chance that the cancers in this family are caused by a single gene mutation?
What is the chance that we can identify the gene mutation in this family with our current knowledge and laboratory techniques?
Cancer clustering in a family may be due to genetic and/or environmental factors, or may be coincidental because some cancers are very common in the general population.26 While inherited factors may be the primary cause of cancers in some families, in others, cancer may develop because an inherited factor increases the individual’s susceptibility to environmental carcinogens. It is also possible that members of the same family may be exposed to similar environmental exposures, due to shared geography or patterns in behavior and diet, that may increase the risk of cancer.27 Therefore, it is important to distinguish the difference between a familial pattern of cancer (due to environmental factors or chance) and a hereditary pattern of cancer (due to a shared genetic mutation). Emerging research is also evaluating the role and clinical utility of more common low-penetrance susceptibility genes and single-nucleotide polymorphisms (SNPs) that may account for a proportion of familial cancers.28
Several models are available to calculate the chance that a woman will develop breast cancer including the Gail and Claus models.29,30 Computer-based models are also available to help determine the chance that a BRCA mutation will be found in a family.31 At first glance, many of these models appear simple and easy to use and it may be tempting to rely on these models, exclusively, to assess cancer risk. However,
each model has its strengths and weaknesses, and the counselor needs to understand the limitations well and know which are validated, which are considered problematic, when a model will not work on a particular patient, or when another genetic syndrome should be considered. For example, none of the existing models are able to factor in other risks that may be essential in hereditary risk calculation (e.g., a sister who was diagnosed with breast cancer after radiation treatment for Hodgkin’s disease).
each model has its strengths and weaknesses, and the counselor needs to understand the limitations well and know which are validated, which are considered problematic, when a model will not work on a particular patient, or when another genetic syndrome should be considered. For example, none of the existing models are able to factor in other risks that may be essential in hereditary risk calculation (e.g., a sister who was diagnosed with breast cancer after radiation treatment for Hodgkin’s disease).
DNA Testing
DNA testing is now available for a variety of hereditary cancer syndromes. However, despite misrepresentation by the media, testing is feasible for only a small percentage of individuals with cancer. DNA testing offers the important advantage of presenting clients with actual risks instead of the empiric risks derived from risk calculation models. DNA testing can be very expensive (full sequencing of the BRCA1/2 genes currently costs >$3,300). All patients being offered BRCA testing should also be offered BRCA rearrangement testing (BART) which looks for large structural rearrangements within these genes.14 It is the clinician’s responsibility to discuss and order this test separately (an additional $700). Importantly, testing should begin in an affected family member, whenever possible. Most insurance companies now cover cancer genetic testing in families where the test is medically indicated.
The results of DNA testing are generally provided in person in a result disclosure session. It is recommended that patients bring a close friend or relative with them to this session who can provide them with emotional support and who can help them listen to and process the information provided.
One of the most crucial aspects of DNA testing is accurate result interpretation. One study found that test results for the hereditary colon cancer syndrome FAP were misinterpreted more than 30% of the time by those ordering the testing.32 More recent data have shown that many medical providers have difficulty interpreting even basic pedigrees and genetic test results.33,34,35 In a survey of over 2,000 physicians, only 13% of internists, 21% of Ob/Gyns, and 40% of oncologists correctly answered four basic knowledge questions about genetic aspects of breast cancer and BRCA testing. This deficiency in knowledge did not necessarily deter them from discussing or ordering testing.5 Misinterpretation of results is now the greatest risk of genetic testing and is very common.36 Interpretation is becoming increasingly complicated as more tests become available. For example, one study demonstrated that approximately 12% of high-risk families who test negative by standard BRCA1 and BRCA2 testing are found to carry a deletion or duplication in one of these genes, or a mutation in another gene.37 This is particularly concerning in an era in which testing companies are canvassing physicians’ offices and are encouraging them to perform their own counseling and testing. The potential impact of test results on the patient and his/her family is great, and therefore, accurate interpretation of the results is paramount. Professional groups have recognized this and have adopted standards encouraging clinicians to refer patients to genetics experts to ensure proper ordering and interpretation of genetic tests. The US Preventive Services Task Force recommends that women whose
family history is suggestive of a BRCA mutation be referred for genetic counseling before being offered genetic testing.38 The American College of Surgeons Commission on Cancer standards include “cancer risk assessment, genetic counseling, and testing services provided to patients either on site or by referral, by a qualified genetics professional.”2
family history is suggestive of a BRCA mutation be referred for genetic counseling before being offered genetic testing.38 The American College of Surgeons Commission on Cancer standards include “cancer risk assessment, genetic counseling, and testing services provided to patients either on site or by referral, by a qualified genetics professional.”2
Results can fall into a few broad categories. It is important to note that a “negative” test result can actually be interpreted in three different ways, detailed in (2), (3), and (4) below:
Deleterious mutation “positive”: When a deleterious mutation in a cancer gene is discovered, the cancer risks for the patient and her family are relatively straightforward. The risks associated with most genes are not precise and should be presented to patients as a risk range.39,40 When a true mutation is found, it is critical to test both parents—whenever possible—to determine from which side of the family the mutation is originating, even when the answer appears obvious.
True negative: An individual does not carry the deleterious mutation found in her family which ideally has been proven to segregate with the cancer family history. In this case, the patient’s cancer risks are usually reduced to the population risks.
Negative: A mutation was not detected and the cancers in the family are not likely to be hereditary based on the personal and family history assessment. For example, a patient is diagnosed with breast cancer at age 38 and comes from a large family with no other cancer diagnoses and relatives who died at old ages of other causes.
Uninformative: A mutation cannot be found in affected family members of a family in which the cancer pattern appears to be hereditary; there is likely an undetectable mutation within the gene, or the family carries a mutation in a different gene. If, for example, the patient developed breast cancer at age 38 has a father with breast cancer and paternal aunt who developed breast and ovarian cancers before age 50, a negative test result would be almost meaningless. It would simply mean that the family has a mutation that could not be identified with our current testing methods or a mutation in another cancer gene. The entire family would be followed as high risk.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree