Genetic Testing by Cancer Site: Pancreas
Jennifer E. Axilbund
Elizabeth A. Wiley
It is estimated that 5% to 10% of pancreatic cancer (adenocarcinoma) is familial,1,2 and individuals with a family history of pancreatic cancer are at greater risk of developing pancreatic cancer, themselves.3 Although there is evidence of a major pancreatic cancer susceptibility gene,4 it remains elusive. Therefore, the majority of families with multiple cases of pancreatic cancer do not have an identifiable causative gene or syndrome, making risk assessment and counseling challenging. However, a subset of pancreatic cancer is attributable to known inherited cancer predisposition syndromes (Table 11.1).
BRCA2
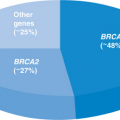
The BRCA2 gene is associated with hereditary breast and ovarian cancer syndrome, and often presents as premenopausal breast cancer, ovarian cancer, and/or male breast cancer. The Breast Cancer Linkage Consortium5 reported a 3.5-fold (95% confidence interval [CI], 1.9 to 6.6) increased risk of pancreatic cancer in BRCA2 gene mutation carriers. Subsequent studies in the United Kingdom and the Netherlands showed a relative risk of 4.1 and 5.9, respectively.6,7 In a US-based study, 10.9% (17/156) of families with a BRCA2 mutation reported a family history of pancreatic cancer. The median ages at diagnosis for males and females were 67 and 59 years, respectively, which differed statistically from the SEER (Surveillance, Epidemiology and End Results) database (70 years old for males and 74 years old for females; P = 0.011).8 Although genotype–phenotype data remain sparse, the BRCA2 K3326X variant was found in 5.6% (8/144) of familial pancreatic cancer patients compared with 1.2% (3/250) of those with sporadic pancreatic cancer (odds ratio [OR], 4.84; 95% CI, 1.27 to 18.55; P < 0.01).9
Approximately, 17% of pancreatic cancer patients who have at least two additional relatives with pancreatic cancer carry deleterious mutations in the BRCA2 gene.10 Estimates for the prevalence of BRCA2 mutations with two first-degree relatives with pancreatic cancer are 6% to 12%,11,12 and BRCA2 mutations also explain a portion of
apparently sporadic pancreatic cancers.13 However, prevalence varies between populations. Six (4.1%) of one hundred forty-five Ashkenazi Jews with pancreatic cancer were found to have a deleterious BRCA2 mutation when compared with cancer-free controls (OR, 3.85; 95% CI, 2.1 to 10.8; P = 0.007), although no differences were noted in age at diagnosis or clinical pathologic features.14 An earlier, smaller study
found a deleterious BRCA2 mutation in 3 (13%) of 23 Ashkenazi Jews with pancreatic cancer, unselected for family history.15 Among Ashkenazi Jewish probands with breast cancer who reported a family history of pancreatic cancer, 7.6% (16/211) had a BRCA2 mutation.16 By comparison, no BRCA2 mutations were found in studies of pancreatic cancer in Korea or Italy.17,18
apparently sporadic pancreatic cancers.13 However, prevalence varies between populations. Six (4.1%) of one hundred forty-five Ashkenazi Jews with pancreatic cancer were found to have a deleterious BRCA2 mutation when compared with cancer-free controls (OR, 3.85; 95% CI, 2.1 to 10.8; P = 0.007), although no differences were noted in age at diagnosis or clinical pathologic features.14 An earlier, smaller study
found a deleterious BRCA2 mutation in 3 (13%) of 23 Ashkenazi Jews with pancreatic cancer, unselected for family history.15 Among Ashkenazi Jewish probands with breast cancer who reported a family history of pancreatic cancer, 7.6% (16/211) had a BRCA2 mutation.16 By comparison, no BRCA2 mutations were found in studies of pancreatic cancer in Korea or Italy.17,18
Table 11.1 Inherited Cancer Predisposition Syndromes that Increase the Risk for Pancreatic Cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
BRCA1
Similar to BRCA2, mutations in BRCA1 are associated with markedly increased risk for premenopausal breast cancer and ovarian cancer. The Breast Cancer Linkage Consortium reported a 2.26-fold (95% CI, 1.26 to 4.06) increased risk of pancreatic cancer in families with a BRCA1 mutation,19 and Brose et al.20 estimated a threefold higher lifetime risk. However, more recently, Moran et al.6 in the United Kingdom found no elevation in pancreatic cancer risk in 268 families with a known BRCA1 mutation. A US-based study reported that 11% (24/219) of their families with a BRCA1 mutation had at least one individual with pancreatic cancer, with median ages at diagnosis of 59 years for males and 68 years for females. Again, this was significantly younger than reported in the SEER database (P = 0.0014).8 Molecularly, Al-Sukhni et al.21 evaluated pancreatic tumors from seven known BRCA1 mutation carriers and found loss of heterozygosity of BRCA1 in five (71%), with confirmed loss of the wild-type allele in three of the five compared with only one (11%) of nine sporadic controls. This suggests that BRCA1 germline mutations do, in fact, predispose to pancreatic cancers in at least some individuals.
Familial breast cancer registries in the United States and Israel have evaluated the mutation status of families that reported pancreatic cancer in addition to breast cancer and ovarian cancer. In the US study of 19 families with breast, ovarian, and pancreatic cancer, 15 carried a deleterious mutation in BRCA1 and 4 in BRCA2,22 whereas the Israeli study reported an equal number of BRCA1 and BRCA2 families.23
Another study, specifically of Ashkenazi Jewish families, reported a BRCA1 mutation in 7% of probands with breast cancer who also had a family history of pancreatic cancer,16 which was, again, equal to the prevalence of BRCA2 mutations. Thus, within the Ashkenazi Jewish population, BRCA1 and BRCA2 mutations may contribute more equally to risk in families with both breast and pancreatic cancer. However, these studies all examined cohorts of families selected because of clustering of breast and/or ovarian cancer with pancreatic cancer. When families were selected on the basis of familial pancreatic cancer, alone, BRCA1 mutations were less prevalent. Zero of sixty-six families with three or more cases of pancreatic cancer had a deleterious BRCA1 mutation, including those who also reported a family history of breast and/or ovarian cancer.24 Evaluation of Ashkenazi Jewish patients ascertained on the basis of pancreatic cancer, alone, showed a 1.3% (2/145) prevalence of BRCA1 mutations.14 Therefore, BRCA1 may explain a small subset of families showing a clustering of pancreatic cancer with breast and/or ovarian cancer, but is unlikely to explain most families with site-specific pancreatic cancer.
PALB2
PALB2 (partner and localizer of BRCA2) was recognized as the FANCN gene in 2007, and biallelic mutation carriers develop Fanconi anemia.25,26 Monoallelic mutation carriers were shown to be at increased risk for breast cancer (relative risk [RR], 2.3; 95% CI, 1.4 to 3.9).27 Prevalence of PALB2 mutations among familial breast cancer cases is low across ethnicities; PALB2 mutations are relatively nonexistent in breast cancers in the Irish and Icelandic populations and are found in approximately 1% of Italians, US African Americans, Chinese, and Spanish breast cancer families, and in 2% of young South African breast cancer patients.28,29,30,31,32,33,34,35 Analysis of 1,144 US familial breast cancer cases found a PALB2 mutation in 3.4% (33/972) of non-Ashkenazi Jews and none (0/172) of Ashkenazi Jews. The estimated risk for breast cancer was 2.3-fold by the age of 55 years (95% CI, 1.5 to 4.2) and 3.4-fold by the age of 85 years (95% CI, 2.4 to 5.9). There was also a fourfold risk for male breast cancer (P = 0.0003) and a sixfold risk for pancreatic cancer (P = 0.002).36 Among French Canadian women with bilateral breast cancer, a PALB2 mutation was found in 0.9% (5/559) compared with none of 565 women with unilateral breast cancer (P = 0.04), and first-degree relatives of PALB2 mutation carriers had a 5.3-fold risk for breast cancer (95% CI, 1.8 to 13.2).37
PALB2 founder mutations have been identified in several populations, including the c.2323 C > T (Q775X) mutation in French Canadians.38 Another example is the Finnish founder mutation c.1592delT. This mutation was found in 2.7% (3/113) of familial breast and/or breast/ovarian cancer families compared with 0.2% (6/2,501) of controls (OR, 11.3; 95% CI, 1.8 to 57.8; P = 0.005).39 One percent (18/1,918) of breast cancer cases, unselected for family history, also had this founder mutation. The hazard ratio for breast cancer was estimated at 6.1 (95% CI, 2.2 to 17.2; P = 0.01), with a penetrance of 40% by the age of 70 years.40