Genetic Testing by Cancer Site: Stomach
Nicki Chun
James M. Ford
Gastric cancer encompasses a heterogeneous collection of etiologic and histologic subtypes associated with a variety of known and unknown environmental and genetic factors. It is a global public health concern, accounting for 700,000 annual deaths worldwide and currently ranking as the fourth leading cause of cancer mortality, with a 5-year survival of only 20%. The incidence and prevalence of gastric cancer vary widely with Asian/Pacific regions bearing the highest rates of disease.
Recent and rapid advances in molecular genetics have provided an understanding of the cause for many inherited cancer syndromes, offering possibilities for individual genetic testing, family counseling, and preventive approaches. For most cancer syndromes, however, not every individual tested is found to have inherited a germline mutation in a candidate gene, suggesting additional uncharacterized alterations in other genes that result in similar outcomes. Nevertheless, the ability to genetically define many individuals and families with inherited cancer syndromes allows for a multidisciplinary approach to their management, often including consideration of surgical and medical preventive measures. Without question, such complex management and decision making should be centered in the high-risk cancer genetics clinic, where physicians, genetic counselors, and other health professionals jointly consider optimal management for patients and families at high risk for developing cancer.
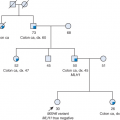
Approximately 3% to 5% of gastric cancers are associated with a hereditary predisposition, including a variety of Mendelian genetic conditions and complex genetic traits. Identifying those gastric cancers associated with an inherited cancer risk syndrome is the purview of cancer genetics clinics. The keystone to any cancer genetics evaluation is a complete, three-generation family history. Pedigree analyses suggesting an inherited gastric cancer risk include familiar features such as multiple affected relatives tracking along one branch of the family in an autosomal dominant pattern, young ages at onset, and additional associated malignancies related to an identified syndrome. It is imperative to document the histology of the gastric tumors and other familial cancers as this is the initial node in the decision tree of an inherited gastric cancer syndrome differential. Finally, there are clinical criteria for recognized gastric cancer syndromes published by
expert consensus panels that assist genetic practitioners in assessing both the likelihood of identifying an underlying germline DNA mutation and guide management in the absence of a molecular confirmation. Herein, we review the literature regarding incidence, recurrence risks, and defined gastric cancer genetic syndromes to assist in providing genetic counseling for families affected by gastric cancer.
expert consensus panels that assist genetic practitioners in assessing both the likelihood of identifying an underlying germline DNA mutation and guide management in the absence of a molecular confirmation. Herein, we review the literature regarding incidence, recurrence risks, and defined gastric cancer genetic syndromes to assist in providing genetic counseling for families affected by gastric cancer.
Histologic Definitions and Descriptions
Gastric cancer has traditionally been subtyped pathologically according to Lauren’s1 classification published in 1965 and revised by Carneiro et al.2 in 1995. The four histologic categories include (1) glandular/intestinal, (2) border foveal hyperplasia, (3) mixed intestinal/diffuse, and (4) solid/undifferentiated.
More clinically relevant, the majority of gastric cancers can be subdivided into intestinal type or diffuse type. Diffuse tumors exhibit isolated cells, typically developing below the mucosal lining, often spreading and thickening until the stomach appears hardened into the morphologic designation called “linitis plastica.” Diffuse gastric tumors frequently feature “signet ring cells,” named for the marginalization of the nucleus to the cell periphery due to high mucin content. Intestinal-type gastric tumors more often present as solid masses with atrophic gastritis and intestinal metaplasia at the periphery. The intestinal subtype is seen more commonly in older patients, whereas the diffuse type affects younger patients and has a more aggressive clinical course. The relative proportions of gastric cancer subtypes worldwide are 74% intestinal versus 16% diffuse and 10% other,3 although diffuse gastric cancer is becoming relatively more common in the Western countries. The importance of distinguishing these two main histopathologic types of gastric cancer is highlighted by finding specific genetic changes associated with the different types. For the purposes of genetic counseling, E-cadherin (CDH1) mutations are found exclusively in the diffuse type.4,5,6,7,8 Whereas intestinal-type hereditary gastric cancer families have been identified clinically, no genetic associations have yet been discovered.
As individual molecular profiling of solid tumors becomes more common in the future, we expect classification systems will evolve based on tumor biology more than histology. Advances in deciphering the mechanisms of gene alterations that lead to gastric cancer include gene mutation, amplification, deletion, and epigenetic methylation.9 For example, two recent studies have performed whole-exome sequencing of human gastric tumors and identified a number of known (e.g., p53, PTEN, PIK3CA), but also previously unreported somatic gene mutations and pathway alterations. Both found ARID1A inactivating gene mutations in the majority of microsatellite-instable tumors, a member of the SWI-SNF chromatin remodeling family.10,11 However, whether any of these somatic gene alterations are found to confer cancer risk when mutated in the germline remains to be determined.
Etiology
Analogous to other common cancers, a host of factors are implicated as causes of gastric cancer. Widely diverse geographical disparities suggest both environmental
and genetic contributions. Furthermore, a strong association with endemic Helicobacter pylori carrier rates implicates infection as a major risk factor. There are likely to be a host of factors contributing to the development of most gastric cancers.
and genetic contributions. Furthermore, a strong association with endemic Helicobacter pylori carrier rates implicates infection as a major risk factor. There are likely to be a host of factors contributing to the development of most gastric cancers.
Environmental Risk Factors
Geographic variations in gastric cancer rates have prompted investigations of shared diet and lifestyle variables. Gastric cancer is correlated with the chronic ingestion of pickled vegetables, salted fish, excessive dietary salt, and smoked meats and with smoking.12,13,14,15,16 Fruits and vegetables may have a protective effect. The influence of environmental factors as causes of gastric cancer is highlighted by declining rates of intestinal gastric cancer among immigrants from high-incident countries to low-incident countries.
Infectious Risk Factors
H. pylori infection is endemic in the Asian-Pacific basin.17 Transmission routinely occurs through family contacts in childhood and leads to atrophic gastritis.18,19 As evidenced by high indigenous infection rates, H. pylori is insufficient to singularly cause gastric cancer, suggesting complex interactions between virus and host genetic backgrounds. However, H. pylori species are consistently implicated as a major risk factor primarily associated with intestinal-type gastric cancer. Studies in a variety of high- and low-risk populations have found odds ratios ranging from 2.56 to 6 for noncardia gastric cancer.20
Epstein-Barr virus has recently been implicated in about 10% of gastric carcinoma worldwide or an estimated 80,000 cases annually. Epstein-Barr virus–associated gastric cancer shows some distinct clinicopathologic characteristics, such as male predominance, predisposition to the proximal stomach, and a high proportion in diffuse-type gastric carcinomas. Mechanistically, Epstein–Barr virus gastric tumors display epigenetic promoter methylation of many cancer-related genes, causing downregulation of their expression.21
Genetics
Five to ten percent of gastric cancer is associated with strong familial clustering and attributable to genetic factors. Shared environmental factors account for the majority of familial clustering of the intestinal type; however, approximately 5% of the total gastric cancer burden is thought to be due to germline mutations in genes causing highly penetrant, autosomal dominant gastric cancer risk of both intestinal and diffuse subtypes. We review the definitions of hereditary gastric cancer families and recognize genetic syndromes associated with increased gastric cancer risk.
Epidemiology of Gastric Cancer
Gastric cancer is now the fourth most common malignancy worldwide, with rates having fallen steadily since 1975 when global statistics were first compared. The
incidence and prevalence of gastric cancer vary widely among world populations. High-risk countries (reported incidence × 100,000 per year) include Korea (41.4), China (41.3), Japan (31.1), Portugal (34.4), and Colombia (20.3). Intermediate-risk countries include Malaysia, Singapore, and Taiwan (11 to 19), whereas low-risk areas include Thailand (8), Northern Europe (5.6), Australia (5.4), India (5.3), and North America (4.3). More than 70% of cases occur in developing countries, and men have roughly twice the risk of women.22 In 2008, estimates of gastric cancer burden in the United States were 21,500 cases (13,190 men and 8,310 women) and 10,880 deaths.23 The median age at diagnosis for gastric cancer is 71 years, and 5-year survival is approximately 25%.24 Only 24% of stomach cancers are localized at the time of diagnosis, 30% have lymph node involvement, and another 30% have metastatic disease. Survival rates are predictably higher for those with localized disease, with corresponding 5-year survival rates of 60%.
incidence and prevalence of gastric cancer vary widely among world populations. High-risk countries (reported incidence × 100,000 per year) include Korea (41.4), China (41.3), Japan (31.1), Portugal (34.4), and Colombia (20.3). Intermediate-risk countries include Malaysia, Singapore, and Taiwan (11 to 19), whereas low-risk areas include Thailand (8), Northern Europe (5.6), Australia (5.4), India (5.3), and North America (4.3). More than 70% of cases occur in developing countries, and men have roughly twice the risk of women.22 In 2008, estimates of gastric cancer burden in the United States were 21,500 cases (13,190 men and 8,310 women) and 10,880 deaths.23 The median age at diagnosis for gastric cancer is 71 years, and 5-year survival is approximately 25%.24 Only 24% of stomach cancers are localized at the time of diagnosis, 30% have lymph node involvement, and another 30% have metastatic disease. Survival rates are predictably higher for those with localized disease, with corresponding 5-year survival rates of 60%.
The worldwide decline in the incidence of gastric cancer has been attributed to modifications in diet, improved food storage and preservation, and decreased H. pylori infection. Fresh fruit and vegetable consumption, refrigeration, decreased urban crowding, and improved living conditions have reduced H. pylori exposure and carrier rates. By contrast, the incidence of diffuse-type gastric cancer is stable, and in North America, it may even be increasing.16,25,26,27
Familial Gastric Cancer
Shared environmental factors, such as diet and H. pylori infection, account for the majority of familial clustering of the intestinal type of gastric cancer, with no known causative germline variants. However, few nongenetic risks for diffuse gastric cancer have been identified, supporting a larger role for hereditary factors. Approximately 5% of the total gastric cancer burden is thought to be due to germline mutations in genes causing a highly penetrant, autosomal dominant predisposition. The International Gastric Cancer Linkage Consortium (IGCLC) has redefined genetic classification of familial intestinal gastric cancer to reflect the background incidence rate in a population (Table 12.1).
Thus, countries with high incidence of intestinal-type gastric cancer (China, Korea, Japan, Portugal) use criteria analogous to the Amsterdam criteria invoked for Lynch syndrome:
At least three relatives with intestinal gastric cancer, one a first-degree relative of the other two,
at least two successive generations affected, and
gastric cancer diagnosed before the age of 50 years in at least one individual.
In countries with a low incidence of intestinal-type gastric cancer (United States, United Kingdom):
At least two first-/second-degree relatives affected by intestinal gastric cancer, one diagnosed before the age of 50 years; or
three or more relatives with intestinal gastric cancer at any age.
Table 12.1 Clinical Criteria for CDH1 Testing Defined by IGCLC 2010 | ||
|---|---|---|
|
Familial intestinal gastric cancer families are similarly prevalent as familial diffuse gastric cancer families, yet a germline genetic defect underlying the disease remains yet to be identified.28 Hemminki et al.29 reported Swedish data on all available types of cancer in first-degree relatives by both parent and sibling probands. The relative risks (RRs) for gastric cancer were greater than 3 for siblings with any relative with gastric cancer and greater than 5 when a sibling was younger than 50 years. Shin et al.30 assessed 428 gastric cancer subjects and 368 controls in Korea for the risk of gastric cancer in first-degree relatives and found an RR of 2.85 with one first-degree relative and greater than 5 in a first-degree relative with H. pylori and a positive family history. Therefore, in the high-incident countries of Japan and Taiwan, population screening for gastric cancer has greatly enhanced early detection, leading to 5-year survival rates of greater than 90%.31
Hereditary Diffuse Gastric Cancer
In 1999, the first IGCLC defined hereditary diffuse gastric cancer (HDGC) as families with (1) two cases diffuse gastric cancer in first-/second-degree relatives with one younger than 50 years, and (2) three cases diffuse gastric cancer at any age.32 The first clear evidence for a gastric cancer susceptibility genetic locus was the identification in 1998 of a germline inactivating mutation in the gene encoding for E-cadherin (CDH1), in a large, five-generation Maori family from New Zealand with 25 kindred with early-onset diffuse gastric cancer.33 The age at diagnosis of gastric cancer ranged upward from 14 years, with the majority occurring in individuals younger than 40 years. The pattern of inheritance of gastric cancer was consistent with an autosomal dominant susceptibility gene with incomplete penetrance. Similar reports of CDH1 mutations in widely diverse HDGC cohorts from Asia, Europe, and North
America followed soon thereafter.34,35,36,37,38,39 Germline CDH1 mutations have been found to be associated with approximately 30% of families with HDGC, with a lifetime risk for gastric cancer of greater than 80%, and up to 60% risk for female carriers developing lobular breast cancer.40 To date, CDH1 is the only gene implicated in HDGC. Worldwide, about 100 CDH1 mutation–positive families have been reported.41
America followed soon thereafter.34,35,36,37,38,39 Germline CDH1 mutations have been found to be associated with approximately 30% of families with HDGC, with a lifetime risk for gastric cancer of greater than 80%, and up to 60% risk for female carriers developing lobular breast cancer.40 To date, CDH1 is the only gene implicated in HDGC. Worldwide, about 100 CDH1 mutation–positive families have been reported.41
E-Cadherin Mutations and Gastric Cancer
The E-cadherin gene coding sequence gives rise to a mature protein consisting of three major domains, a large extracellular domain (exons 4 to 13) and smaller transmembrane (exons 13 to 14) and cytoplasmic domains (exons 14 to 16). As in other autosomal dominant cancer predisposing genes, only one CDH1 allele is mutated in the germline, and the majority of genetic changes lead to truncation of the protein, with mutations distributed throughout the gene’s 2.6 kb of coding sequence and 16 exons without any apparent hotspots. Somatic CDH1 mutations have been identified in about half of sporadic diffuse gastric cancers, but occur rarely in intestinal gastric cancer. CDH1 encodes the calcium-dependent cell-adhesion glycoprotein E-cadherin. E-cadherin is a transmembrane protein that connects to the actin cytoskeleton through a complex with catenin proteins.5,42 Functionally, E-cadherin impacts maintenance of normal tissue morphology and cellular differentiation. With regard to HDGC, it is believed that CDH1 acts as a tumor suppressor gene, with mutation of CDH1 leading to loss of cell adhesion, proliferation, invasion, and metastasis.43
Genetic Testing for HDGC
At the second meeting of the IGCLC in 2010, HDGC guidelines44 were extended to recommend CDH1 genetic testing to families with
two cases of gastric cancer in which one case is histopathologically confirmed as diffuse and younger than 50 years,
families with both lobular breast cancer and diffuse gastric cancer, with one diagnosed younger than 50 years, and
probands diagnosed with diffuse gastric cancer younger than 40 years, with no family history of gastric cancer.
Using the initial IGCLC criteria for HDGC, CDH1 mutation testing yielded a detection rate of 30% to 50%.45 Interestingly, a pattern began to emerge of lower CDH1 mutation rates among HDGC families in high gastric cancer incidence populations and higher rates in low-incident countries.46,47 Other reports suggest that the rate of CDH1 mutations in isolated cases of diffuse gastric cancer younger than 35 years is similar in both low- and high-risk countries hovering at around 20%.48
Approximately 50% to 70% of clinically diagnosed HDGC families have no identifiable genetic mutation. Multiple candidate loci have been investigated without
identifying causative mutations that would account for the large number of non-CDH1 HDGC families.49,50,51 Huntsman’s group has published a report of multiplex ligation-dependant probe amplification-based exon duplication/deletion studies performed on 93 non-CDH1 families and found 6.5% carried large genomic deletions bringing the detection rate up to 45.6% in their cohort of 160 families.52
identifying causative mutations that would account for the large number of non-CDH1 HDGC families.49,50,51 Huntsman’s group has published a report of multiplex ligation-dependant probe amplification-based exon duplication/deletion studies performed on 93 non-CDH1 families and found 6.5% carried large genomic deletions bringing the detection rate up to 45.6% in their cohort of 160 families.52
As CDH1 mutation families were identified, data on these families provided the foundation for genetic counseling information. Initially, the cumulative risk of gastric cancer by the age of 80 years in HDGC families was initially estimated as 67% for men and 83% for women. The age at onset shows marked variation between and within families. The median age at onset in the 30 Maori CDH1 mutation carriers who developed gastric cancer was 32 years, significantly younger than the median age of 43 years in individuals with gastric cancer from other ethnicities.53 More recent reports of the lifetime risks of diffuse gastric cancer suggest greater than 80% in both men and women by the age of 80 years.48,54
The lifetime risk for lobular breast cancer among female CDH1 carriers, originally estimated to be in the range of 20% to 40%, now approaches 60% with an average age of 53 years at the time of diagnosis.36,54,55 Of note, CDH1 mutations have been seen in up to 50% of sporadic lobular breast cancer. Pathologic similarities between diffuse gastric and lobular breast carcinomas such as high mucin content with associated signet ring features and loss of E-cadherin on immunohistochemistry hint at a common molecular mechanism.56,57 To evaluate the CDH1 carrier rate in women with lobular breast cancer without a family history of diffuse gastric cancer, a multicenter study of 318 women with lobular-type breast cancer diagnosed before the age of 45 years and known to be BRCA1/2-negative were sequenced for CDH1 mutations. Only four possibly pathogenic mutations were identified for a rate of 1.3%, suggesting CDH1 is a rare cause of early lobular cancer without associated gastric cancer family history.58
Signet ring colon cancer has been reported in two families with germline CDH1, but no screening guidelines have been suggested.45,59 Nonsyndromic cleft lip and/or palate was reported in seven individuals from three families in the Netherlands and in four individuals from two families in France. There is speculation that defects in the cell-adhesion role of E-cadherin may contribute to this developmental anomaly, although no association can be drawn from these scant case reports.40,60
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree