Genetic Testing by Cancer Site: Ovary
Scott M. Weissman
Shelly M. Weiss
Anna C. Newlin
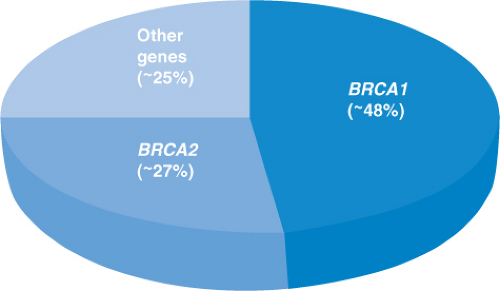
Ovarian cancer is responsible for ∼3% of all cancers among women, and in 2012, there will be ∼22,280 new cases and 15,500 deaths from this cancer; this is the highest death rate for any cancer of the female reproductive system.1 Given this fact, it is of critical importance to identify women who face an elevated risk for ovarian cancer to allow for early detection or prevention. A number of risk factors (e.g., nulliparity, early menarche, late menopause) for ovarian cancer have been identified and reviewed by others.2,3,4 By far, the most important risk factor is family history. Having one first-degree relative with ovarian cancer increases the lifetime risk from 1.4% (average risk) to 5% and at least 7% with two or more first-degree relatives.5 However, in families with two or more cases of ovarian cancer, there may be a hereditary cause for the cancer, which in turn would result into higher lifetime ovarian cancer risks. Historically, it was believed that ∼10% of ovarian cancers were due to an underlying hereditary syndrome, but more recent data indicate that just two syndromes (hereditary breast and ovarian cancer syndrome and Lynch syndrome) account for at least 20% of ovarian cancers, and overall, at least 25% of newly diagnosed cases are due to a hereditary mutation in a single gene6,7,8; this suggests that a much larger proportion of ovarian cancer cases is hereditary in nature than originally thought (Fig. 6.1). This chapter reviews ovarian cancer within the context of known hereditary cancer syndromes. In addition, we address some of the newer genes ovarian cancer has been linked to as clinical genetic testing for some of these genes are quickly becoming available to health care professionals.
Hereditary Breast and Ovarian Cancer Syndrome (The BRCA1 AND BRCA2 GENES)
Hereditary breast and ovarian cancer syndrome due to mutations in the BRCA1 and BRCA2 (BRCA1/2) genes is the most common cause of hereditary ovarian cancer, including fallopian tube and primary peritoneal cancer. Anywhere from 0.125% to 0.20% of the general population carry mutations in BRCA1/2 compared
with 15% of women with a diagnosis of invasive ovarian cancer.6,8,9,10 The contribution of the BRCA1/2 genes to ovarian cancer is even greater in certain ethnicities that have higher BRCA1/2 mutation prevalence rates (e.g., Polish, Ashkenazi Jewish [AJ], French Canadian). For example, the prevalence of BRCA1/2 mutations in individuals of AJ ancestry and women of AJ ancestry with ovarian cancer is ∼2.3% and ∼30% to 40%, respectively.11,12,13 Because of the strong connection between ovarian cancer and BRCA1/2, multiple professional societies and organizations recommend genetic counseling and testing for any woman with ovarian cancer regardless of age at onset or family history (Table 6.1). The current sensitivity of BRCA1/2 genetic testing at the commercial laboratory is ∼90% for identifying mutations in either gene.
with 15% of women with a diagnosis of invasive ovarian cancer.6,8,9,10 The contribution of the BRCA1/2 genes to ovarian cancer is even greater in certain ethnicities that have higher BRCA1/2 mutation prevalence rates (e.g., Polish, Ashkenazi Jewish [AJ], French Canadian). For example, the prevalence of BRCA1/2 mutations in individuals of AJ ancestry and women of AJ ancestry with ovarian cancer is ∼2.3% and ∼30% to 40%, respectively.11,12,13 Because of the strong connection between ovarian cancer and BRCA1/2, multiple professional societies and organizations recommend genetic counseling and testing for any woman with ovarian cancer regardless of age at onset or family history (Table 6.1). The current sensitivity of BRCA1/2 genetic testing at the commercial laboratory is ∼90% for identifying mutations in either gene.
The lifetime risk of developing ovarian cancer differs between the two genes. A number of studies over the years have quantified the lifetime risk20,21,22,23,24,25,26; however, two large meta-analyses suggest an ∼40% and 20% lifetime risk for BRCA1 and BRCA2 mutation carriers, respectively.27,28 In 1997, Gayther et al.,29 in an effort to determine whether there were any genotype–phenotype correlations associated with BRCA2 mutations, identified a region in exon 11 of BRCA2 between nucleotides 3,035 and 6,629 that appeared to confer a further increased risk of ovarian cancer; they coined this region the “ovarian cancer cluster region” (OCCR). A follow-up study of the OCCR in families from the Breast Cancer Linkage Consortium (which included the families from the study by Gayther et al.29) further refined the OCCR to nucleotides 3,059 to 4,075 and 6,503 to 6,629, but found that the phenotype of increased ovarian cancer may actually be due to a reduced breast cancer risk.30 Regardless, the potential difference in ovarian cancer risk was not significant enough to affect management recommendations. In addition to genotype–phenotype associations, researchers have studied other genetic modifiers (e.g., single-nucleotide
polymorphisms) that can influence ovarian cancer risk in BRCA1/2 mutation carriers, but these data need maturation before testing for genetic modifiers can be used clinically.31,32,33,34
polymorphisms) that can influence ovarian cancer risk in BRCA1/2 mutation carriers, but these data need maturation before testing for genetic modifiers can be used clinically.31,32,33,34
Table 6.1 Societies or Organizations with Position Statements Recommending BRCA1/2 Genetic Counseling and/or Testing Related to Ovarian Cancer | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
The average age at onset of ovarian cancer is between 49 and 53 years for BRCA1 and 55 and 58 years for BRCA2 compared with 63 years in the general population.6,8,35,36,37 Unlike breast cancer, women with a diagnosis of very early-onset ovarian cancer (<40 years) are significantly less likely to harbor BRCA1/2 mutations.8,26,35,38,39 This is in part due to the fact that early-onset ovarian cancers are more likely to be associated with borderline tumors, earlier stages, and more favorable histologic characteristics, none of which are typical of BRCA1/2-related ovarian cancer.40
BRCA1/2-associated ovarian cancers are almost uniformly epithelial in origin and, for the most part, are invasive and nonmucinous; there are case reports of germ cell and stromal tumors in BRCA1/2 mutation carriers. Mucinous and borderline tumors individually account for ∼2% of ovarian tumors identified in BRCA1/2 mutation carriers; this percentage is the same for both prospective and retrospective analyses, which are nicely summarized by Evans et al.41 Compared with sporadic ovarian cancers, BRCA1/2 ovarian cancers are more often of serous histology, higher grade, and solid type, and have intact p53 staining on immunohistochemistry (IHC).39,41,42,43,44 It is important to note that other histologic findings are seen in mutation carriers (e.g., endometrioid, clear cell, papillary), and one study found more giant cell-type cancers in BRCA1 mutation carriers compared with controls.42 Several smaller studies have found that BRCA1/2-related ovarian cancers have a better prognosis compared with ovarian cancers in nonmutation carriers.39,45,46,47,48 This finding seems to have been confirmed as a recent large pooled analysis of 26 observational studies comparing 3,879 BRCA1/2 ovarian cancers and 2,666 noncarriers found the 5-year survival for BRCA2 carriers was 52%, 44% for BRCA1 carriers, and 36% for noncarriers.44 The survival difference remained after adjusting for age at diagnosis, stage, histology, and grade.
One potential reason for the differences in survival may be that BRCA1/2-related ovarian cancers respond better to platinum-based agents.45,48 BRCA1/2 repair DNA damage through homologous recombination and platinum agents are particularly active in cells deficient in homologous recombination.48,49 It is through this pathway that another class of drugs called poly(ADP-ribose) polymerase (PARP) inhibitors has been developed to help treat BRCA1/2-related cancers. Unlike platinum agents targeting homologous recombination, PARP inhibitors block repair of single-strand DNA breaks through base excision repair, which in turn can lead to double-strand breaks that cannot be repaired by BRCA1/2-deficient tumor cells at the same time sparing normal cells.50,51,52 A number of phase I and phase II trials have been reported, and clinical trials continue to study PARP inhibitors in both ovarian and breast cancers.53,54,55,56
Identifying women who have a BRCA1/2 mutation would ideally lead to women either being diagnosed with ovarian cancer at earlier stages or preventing ovarian cancer altogether. When counseling women who have tested positive for a BRCA1/2 mutation, it is these central tenets, ovarian cancer screening versus prevention, that guide discussions. Current National Comprehensive Cancer Network (NCCN) guidelines for managing ovarian cancer risk include the recommendation for risk-reducing bilateral salpingo-oophorectomy (RRSO) between the ages of 35 and 40 years when childbearing is complete; for women not choosing RRSO, transvaginal ultrasound, and CA-125 are recommended every 6 months starting at age 30 years or 5 to 10 years before the earliest age at onset of ovarian cancer in the family.17 Surgical prevention is recommended over screening for two main reasons. First, many women can receive a dual risk reduction with one surgery. RRSO has been shown to reduce ovarian cancer risk by 80% to 95%,57,58,59,60 and breast cancer risk by 50% (for premenopausal women) in BRCA1/2 mutation carriers.59,60,61,62 Anywhere from 2.5% to 17%63




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree