Genetic Testing by Cancer Site: Breast
Kristen M. Shannon
Anu Chittenden
Women in the United States have a 12% lifetime risk of developing breast cancer.1 Although only about 5% to 10% of all cases of breast cancer are attributable to a highly penetrant cancer predisposition gene, individuals who carry a mutation in one of these genes have a significantly higher risk of developing breast cancer, as well as other cancers, over their lifetime compared with the general population. The ability to distinguish those individuals at high risk allows health care providers to intervene with appropriate counseling and education, surveillance, and prevention with the overall goal of improved survival for these individuals. This chapter focuses on the identification of patients at high risk for breast cancer and provides an overview of the clinical features, cancer risks, causative genes, and medical management for the most clearly described hereditary breast cancer syndromes.
Identification of High-Risk Individuals
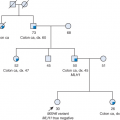
An accurate and comprehensive family history of cancer is essential for identifying individuals who may be at risk for inherited breast cancer. As with any family history, it is important to gather a three-generation family history with information on both maternal and paternal lineages.2,3 Particular focus should be on individuals with malignancies (affected), but those family members without a personal history of cancer (unaffected) should also be included. It is also important to include the presence of nonmalignant findings in the proband and family members, as some inherited cancer syndromes have other physical characteristics associated with them (e.g., trichilemmomas with Cowden syndrome [CS]).
When taking the family history, the accuracy of the information obtained from an individual patient should be considered. Many factors can influence an individual’s knowledge of his/her family history, and errors in the reporting of family history have been documented.4,5 A recent study indicates that individuals are often confident that a family member has had cancer but are typically unsure of the details surrounding that diagnosis.6,7 Reports of breast cancer tend to be accurate, whereas reports of
ovarian cancer are less trustworthy.8,9 It is important to note that family histories can change over time, with clinically relevant diagnoses arising in family members especially between the ages of 30 and 50 years.10 Finally, the physical examination of the proband and family members can be incredibly helpful in the identification of some inherited breast cancer syndromes, such as CS.
ovarian cancer are less trustworthy.8,9 It is important to note that family histories can change over time, with clinically relevant diagnoses arising in family members especially between the ages of 30 and 50 years.10 Finally, the physical examination of the proband and family members can be incredibly helpful in the identification of some inherited breast cancer syndromes, such as CS.
Genetic Testing
Although some published guidelines for genetic testing exist, much of the time the decision to offer genetic testing is based on clinical judgment. The National Comprehensive Cancer Network (NCCN) provides guidelines for individuals whom should be offered genetic testing for some of the genes mentioned in this text (NCCN 2011). In the end, however, it is up to the individual provider’s judgment as to whether genetic testing is indicated.
Genetic testing for breast cancer susceptibility is rapidly changing. The classic method includes pursuing genetic testing for individual cancer predisposition gene(s) that the clinical suspects may be the cause of breast cancer in the family. In this scenario, finding the appropriate laboratory to perform the testing is very important because laboratory techniques (as well as sensitivity of the technique) vary. Most genetic testing includes sequencing of the gene in question. However, there are emerging data that suggest deletion/duplication studies are imperative for genetic testing as the mutational spectra include various rare, yet important genomic rearrangements.11
Recent changes in genetic testing and specifically the advent of next-generation sequencing tests, have led various genetic testing companies to establish “panel” testing for multiple breast cancer susceptibility genes. In this scenario, up to 14 different breast cancer susceptibility genes are analyzed from 1 blood specimen. These genes vary in clinical significance from the very highly penetrant breast cancer susceptibility gene TP53 to the low penetrant breast cancer gene CHEK2. How this testing will evolve and the role it will play in clinical care remains to be seen.
BRCA1 and BRCA2
Description
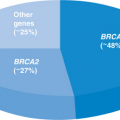
Mutations in the BRCA1 and BRCA2 genes give rise to the “classic” inherited breast cancer syndrome “hereditary breast and ovarian cancer (HBOC) syndrome.” The vast majority of cases of HBOC are due to mutations in the BRCA1 and BRCA2 genes,12,13 which were cloned in 1994 and 1995, respectively.14,15 BRCA1 and BRCA2 mutations are rare in most populations, occurring in approximately 1 of 400 individuals, but much more common in the Ashkenazi Jewish population in which 1 of 40 individuals carries 1 of 3 main disease-causing mutations: 2 in BRCA1 (185delAG and 5382insC) and the 6174delT mutation in BRCA2.16,17 Other founder mutations have been identified, but the utility of these in the United States population is minimal.18,19
There has been a great deal of research into the tumor biology associated with BRCA1/2 mutation carriers. BRCA2-associated breast cancers are similar in phenotype and clinical behavior in comparison to sporadic cancers.20,21 BRCA1-related breast cancers are often of higher histologic grade, show an excess of medullary histopathology, and are more likely than sporadic tumors to be “triple negative” (i.e., estrogen receptor–negative, progesterone receptor–negative, and are less likely to demonstrate HER2/neu overexpression).22 Serous papillary ovarian carcinoma is a key feature of hereditary cancers in BRCA1 mutation carriers; it is less common in BRCA2 carriers. Endometrioid and clear-cell subtypes of ovarian cancer have been observed,23 but borderline ovarian tumors do not seem to be a part of the phenotype.24 Both primary tumors of the fallopian tubes and peritoneum occur with increased frequency in mutation carriers.25 The prognosis of ovarian cancer in BRCA1 and BRCA2 carriers is better than age-matched controls.23,26,27
Identifying BRCA12 Carriers
Identifying those individuals at highest risk for harboring a mutation in BRCA1 or BRCA2 is of utmost importance so that they can benefit from surveillance and prevention options. There exist various models designed to estimate the likelihood of identifying a mutation in the BRCA1 or BRCA2 gene13,28,29,30,31; these models have strengths and limitations that health care providers need to be familiar with to use and interpret them appropriately.32,33,34 The BRCAPRO model, likely the most often used in clinical cancer genetics, estimates the probability that an individual is a carrier of a BRCA mutation using family history and Bayes’ theorem.28 It is important when using these risk models to understand the limitations of these risk calculations and to place risk estimates into the appropriate context. It is important to note that risk estimates calculated by different models may vary, a factor that complicates the use of quantitative thresholds for making screening recommendations.35 The health care provider should use clinical judgment in conjunction with estimates from models to provide the most precise risk assessment for an individual patient.
Cancer Risks
The penetrance associated with mutations in BRCA1 and BRCA2 remains an active area of research. The risks of developing specific cancers can be found in Table 5.1. The range of breast cancer risk is influenced by the population under study: Higher risk estimates have come from studies with affected families and somewhat lower risk estimates from studies in populations. Also, the risk of ovarian cancer is not the same for all BRCA2 mutations, with mutations in the central ovarian cancer cluster region conferring a higher lifetime risk.42 Other factors, such as birth cohort, oral contraceptive use, age at first pregnancy, and exercise, have all been shown to influence penetrance risk in populations.36 There has been a report of increased risk of gallbladder and bile duct cancer, stomach cancer, and melanoma with BRCA2 mutation, none of which seem to be clinically actionable.37,43
Table 5.1 BRCA1/2 Cancer Risks | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Management
The current recommendations for the screening of women at risk for HBOC is based on the best available evidence and is expected to change as more specific features of BRCA1– and BRCA2-related disease become available. The current screening recommendations for women are listed in Table 5.2.
Risk-reduction mastectomies are an appropriate consideration for women at the highest hereditary risk for breast cancer. Studies have shown a 90% to 95% reduction in breast cancer risk following prophylactic mastectomy.44,45,46,47 The evidence for the use of tamoxifen or raloxifene as a chemopreventive agent in BRCA carriers is limited; however, tamoxifen has been shown to reduce the risk of contralateral breast cancers in BRCA carriers.48,49 Two recent studies support the role of risk-reducing salpingo-oophorectomy: The hazard ratio for ovarian cancer for women who underwent prophylactic surgery and that for those who chose close surveillance were 0.15 and 0.04, respectively.50,51 Women should be informed about the potential for the subsequent development of peritoneal carcinomatosis, which has been reported up to 15 years after risk-reducing bilateral salpingo-oophorectomy.25,52 Combination oral contraceptives containing estrogen and progestin result in a protective effect against ovarian cancer in some studies, but not in others.53,54,55
Male BRCA mutation carriers are advised to undergo training in breast self-examination with regular monthly practice and semiannual clinical breast examinations, and workup of any suspected breast lesions is recommended. The NCCN Guidelines also recommend that a baseline mammogram be considered, with an annual
mammogram if gynecomastia or parenchymal/glandular breast density is identified on baseline study.56 The NCCN Guidelines recommend that male BRCA mutation carriers should adhere to the current prostate cancer screening guidelines.56,57
mammogram if gynecomastia or parenchymal/glandular breast density is identified on baseline study.56 The NCCN Guidelines recommend that male BRCA mutation carriers should adhere to the current prostate cancer screening guidelines.56,57
Table 5.2 NCCN Guidelines for Management of BRCA1/2 Carriers | |||
|---|---|---|---|
|
Psychosocial Considerations
The psychosocial needs of BRCA-positive women have been studied fairly widely. Studies have shown that although there is slight worsening of distress symptoms following cancer genetic counseling in BRCA1/BRCA2 mutation carriers, these symptoms were minimal, did not affect everyday life activities, and had almost disappeared at 1-year follow-up.58,59,60,61,62 Approximately, 20% of BRCA1/2 mutation carrier women experience high distress after learning their test result.63,64 Factors that are related to high posttest distress include a high level of pretest anxiety, higher pretest perceived risk, and whether they are opting for prophylactic surgery to reduce their risk.59,63,65 It is important to note, however, that even in women who experienced distress after receipt of genetic test information, women do not “regret” their decision to be tested.66 It has been suggested that health care providers consider including a brief pretest psychological assessment before initiating genetic testing for BRCA1 and BRCA267 so that these women can be targeted for more comprehensive support once test results are available.68
The anxiety-associated symptoms reported by BRCA1/2 carriers include sleeplessness and “bad mood.”60,69,70 One other psychosocial issue reported by single women with BRCA1/2 mutations is that they experience increased urgency at finding a life partner capable of handling the emotional strain of the cancer world and open to pursuing multiple paths toward parenthood.71
Various studies have suggested that existing social support networks are inadequate for BRCA1/2 mutation carriers and that formal services are unavailable or underutilized.66,70,72 To address this lack of formal support services, a retreat for BRCA1/2 carriers that includes educational updates about medical management, genetic privacy, and discrimination and addresses psychological and family issues may provide a valuable opportunity for BRCA carriers and their families to receive updated medical information, share personal experiences, provide and receive support, and change health behaviors.73
Distress in male BRCA carriers has not be studied quite as widely, but one study noted that high distress after disclosure of the result was reported by 1 of 4 male mutation carriers.74
TP53
Description
Germline mutations in the TP53 gene give rise to a disease called Li–Fraumeni syndrome (LFS), which is a rare cancer predisposition syndrome thought to be responsible for ∼1% of breast cancers.75 LFS is often thought of as a hereditary predisposition to cancer in general, involving many tumor types and occurring at any point in an
individual’s lifetime, including childhood. The majority of cases of LFS are due to mutations in the p53 gene.76,77,78,79 The component tumors of LFS include bone sarcomas (primarily osteosarcomas and chondrosarcomas), soft-tissue sarcomas, breast cancer, brain tumors, leukemia, and adrenocortical carcinomas.80 The classic component tumors are thought to account for 63% to 77% of cancer diagnoses in individuals with LFS.80,81,82,83



individual’s lifetime, including childhood. The majority of cases of LFS are due to mutations in the p53 gene.76,77,78,79 The component tumors of LFS include bone sarcomas (primarily osteosarcomas and chondrosarcomas), soft-tissue sarcomas, breast cancer, brain tumors, leukemia, and adrenocortical carcinomas.80 The classic component tumors are thought to account for 63% to 77% of cancer diagnoses in individuals with LFS.80,81,82,83
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree