Essential tools for the practicing gynecologist are an understanding of benign and malignant breast diseases, the ability to detect and diagnose breast cancer, and an appreciation of the various treatment options for the breast cancer patient. In this chapter, benign conditions that can masquerade as malignant, are discussed, as well as the diagnosis and management of in situ and invasive breast cancers.
Detection
Physical Examination
Breast malignancies are usually asymptomatic and are discovered only by physical examination or screening mammography. Any physical findings on a routine breast examination must be recorded in the medical record for future reference.
For the breast and nodal examination, the patient should be examined in both the upright and supine positions. Examination should begin with inspection of the breasts with the patient seated comfortably, arms relaxed at her sides. Differences in symmetry or contour of the breasts should be noted, as well as any skin changes such as edema or erythema. Skin dimpling or nipple retraction may become apparent by having the patient raise her arms above her head then press her hands on her hips (Fig. 16.1). The cervical, supraclavicular, and axillary areas should be palpated for enlarged nodes. With the patient still seated, each breast should be examined by using the nondominant hand to support the breast, while palpating with the dominant hand using the flat portion of the fingers rather than the tips. The upper outer quadrant of the breast up to the clavicle, the axillary tail of Spence, and the axilla should be palpated for possible masses. The nipples should be assessed for nipple discharge.
The patient should then be asked to lie down and raise one arm over her head, while keeping the other arm at her side. The breast examination should commence on the side with the raised arm, and proceed systematically from the clavicle to the costal margin. The manner in which the breast is examined is not as important as consistency and diligence; it is important to choose one method that allows for examination of the entire breast that can be repeated methodically. One technique that many clinicians use is to palpate the breast in enlarging concentric circles. Placement of a pillow or towel beneath the scapula to elevate the side examined is important for women with large, pendulous breasts, which tend to fall laterally, making palpation of the lateral breast challenging.
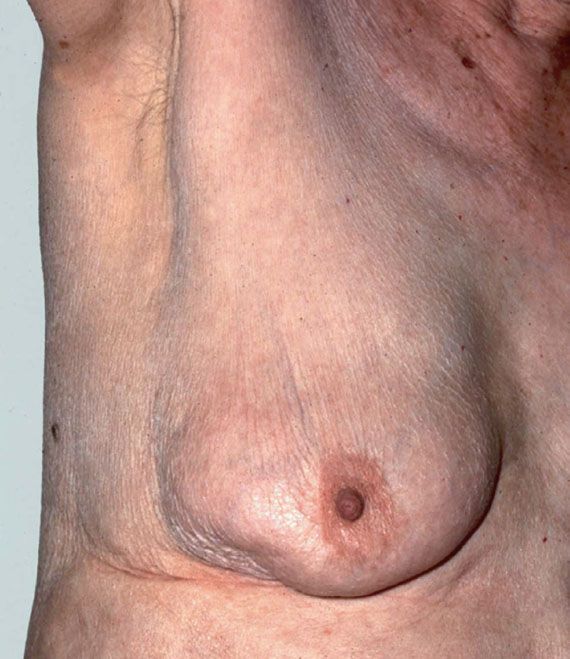
Figure 16.1 Retraction of the skin of the lower, outer quadrant seen only on raising the arm. A small carcinoma was palpable.
The major features in a breast examination to be identified are nodularity, nipple inversion or discharge, skin retraction, tenderness, and dominant masses. Any abnormalities should be documented by identifying the area of concern as the position on the face of a clock, and its distance from the nipple, for example, a right breast lesion at 10 o’clock, 5 cm from the nipple is located in the upper outer quadrant. Many patients, particularly young, premenopausal women, have nodular breast parenchyma. Nodularity is frequently diffuse although it tends to be more prevalent in the upper outer quadrants where there is more breast tissue. Nodules are small, indistinct, and similar in size. Conversely, breast cancers tend to present as nontender, firm masses with unclear margins, often feeling distinct from the surrounding nodularity. Malignant masses may also be fixed to the chest wall (underlying fascia) or to the skin. Not all breast cancers possess these characteristics, so any dominant mass in the breast requires further evaluation.
Around the time of menses, many women have increased nodularity and engorgement of the breast, which occasionally obscures an underlying lesion. If a patient presents to the physician with concern about a mass that she has palpated and the physician cannot confirm the patient’s finding because of breast engorgement, the examination should be repeated after the patient’s menses. Mammography or ultrasonography is a useful adjunct in cases where the patient has palpated a mass, yet the clinician is unable to confirm the finding.
Breast Self-examination
Although there is no evidence to date that breast self-examination (BSE) leads to a decreased mortality rate by diagnosing breast cancers at an early stage, its utilization is still advocated by many organizations (1,2). It is viewed as a surveillance tool for women to heighten awareness of the normal composition of the breast, and to detect changes that may occur. BSE can be useful in detecting interval cancers between screenings. BSE should not be used in isolation; it supplements screening by clinical breast examination (CBE) and mammography.
It is advocated by the American Cancer Society (ACS) and the National Comprehensive Cancer Network (NCCN) that BSE begin early, at age 20 years. Starting BSE at an early age allows women to familiarize themselves with the composition of their breasts, increases awareness of breast cancer surveillance, and establishes a good habit. Women should report any changes to their physicians. As part of the ACS guidelines for the Early Detection of Breast Cancer, CBE should be performed every 3 years starting at age 20, with the option of monthly BSE also starting at age 20. Premenopausal women may find that monthly examinations are most informative during the week after their menses. There are complex reasons why many women do not perform BSE, but reassurance and patient education may encourage women to overcome psychological barriers. In women who have been treated for breast cancer, BSE can be used as a supplemental method to aid in detecting recurrence.
Like CBE, the BSE should begin with visual inspection. A woman should inspect her breasts while standing or sitting before a mirror, looking for asymmetry, nipple retraction, or skin dimpling. Skin dimpling is highlighted by elevation of the arms over the head or by pressing the hands against the hips to contract the chest muscles. While standing or sitting, the woman should carefully palpate her breasts using the finger pads of the opposite hand, initially with light pressure then with increasing firmness. This may be performed while showering with soapy hands to increase the sensitivity of palpation. Finally, she should lie down and again palpate each quadrant of the breast extending into the axilla. A good resource for instructions on BSE can be found on website below: http://www.breastcancer.org/symptoms/testing/types/self_exam/bse_steps.
Breast Imaging
The two most important imaging techniques are mammography and ultrasonography. Magnetic resonance imaging (MRI) may be used as a screening tool in subsets of women at higher risk of breast cancer, and as a diagnostic tool in certain clinical situations.
Screening Mammography
Screening of asymptomatic women for breast cancer has been shown to reduce the death rate by 20–30%, most likely as a result of earlier detection. Half of the reduction seen in breast cancer deaths in the United States has been attributed to screening mammography (3).
Several randomized controlled trials (RCTs) support the use of mammography for breast cancer screening in women older than 50 years of age. Controversy regarding the benefit of screening women aged 40 to 49 years is primarily related to by the lack of relevant RCTs. Accordingly, in 2009, the US Preventive Services Task Forces recommended that “the decision to start regular biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms (4).” When data regarding screening in women aged 40 to 49 years were combined from the various RCTs and analyzed in a meta-analysis, there was a significant reduction of 18% in the breast cancer mortality rate for this group of women (5). The Swedish Two-Country Trial demonstrated a 23% reduction in mortality rate for this group of women with screening mammography and 18 years of follow-up (6). A UK study has shown a survival benefit in younger women equivalent to that seen in women over 50 years (7).
The ACS, American Society of Clinical Oncologists (ASCO), and the American College of Radiology recommend that annual mammographic screening should begin at age 40 for women at normal risk (8), and the National Cancer Institute recommends screening mammography every other year for women 40 and older (9). Additional screening recommendations exist for women at increased risk of breast cancer; these recommendations include more frequent CBE, initiation of screening at ages younger than 40, and using other modalities as adjuncts to mammography, including ultrasound and MRI. There is no established upper age limit for breast cancer screening.
Mammography is the best method of detection for a nonpalpable breast cancer but occasionally misses some palpable and nonpalpable or ultrasonographically detected malignancies. Mammography should not be used in isolation. Neither the technology of mammography nor its interpretation by radiologists is infallible. The basis of any screening program or workup of breast abnormalities is the physical examination. Mammography and CBE complement one another; it is recommended that women undergo CBEs around the time of their regularly scheduled annual mammograms (8). Patients should be cautioned that breast compression can be uncomfortable and that steady compression of the breast is necessary to obtain good images.
In addition to mammography used as a screening tool, there are circumstances in which bilateral mammography is mandatory:
1. In any patient with a dominant mass, even if biopsy is planned, to assess the ipsilateral lesion and to exclude disease in the contralateral breast.
2. In any patient with enlarged axillary or supraclavicular nodes, in order to search for an occult primary breast carcinoma.
3. Before any cosmetic breast operation (augmentation, implant exchange, breast reduction) to rule out occult disease.
Mammographic Abnormalities
Mammography can detect microcalcifications, breast densities, and architectural distortions (Fig. 16.2). To standardize the reporting of mammographic findings, the American College of Radiology developed the Breast-Imaging Reporting and Data System (BI-RADS) classification (10). There are six categories (1–6) for classifying findings, with category 0 representing an incomplete assessment with the need for additional studies (Table 16.1). Additional imaging recommendations may include magnification, spot compression, and ultrasonography. A patient may have a screening mammogram that shows a questionable abnormality, while spot compression views may indicate the finding is completely normal; this would fall into category 1 “negative.”
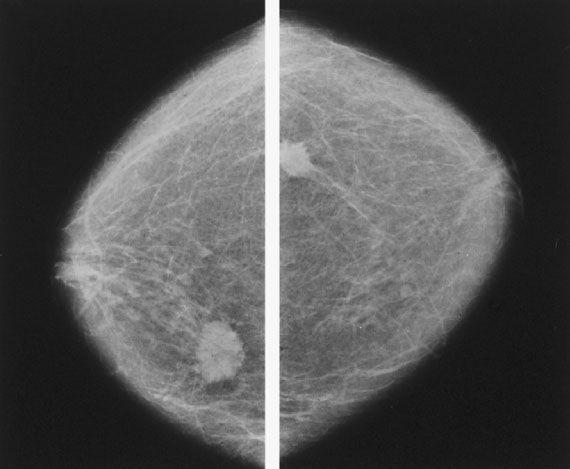
Figure 16.2 Bilateral film screen mammograms showing a typical carcinoma in each breast, illustrating the importance of bilateral mammography in the workup of a clinically apparent mass.
Table 16.1 Mammographic Interpretations and the Breast Imaging Reporting and Data System Classification with Recommendations

Mammographic Findings
Microcalcifications are the most frequent mammographic abnormalities necessitating biopsy. The evaluation of microcalcifications includes review of their size, number, location, distribution, and morphology. Microcalcifications that tend to be benign are smooth, round, solid, or lucent-centered spheres. Tubular or rod-shaped calcifications are often associated with ectatic ducts. If further analysis is required, magnification and spot compression views are the primary techniques used. Calcifications that are classified as benign do not require histologic confirmation. Of concern are calcifications that vary in size and shape (pleomorphic). Most calcifications in breast cancers form in the intraductal portion of the breast and are small and irregular, with varying levels of maturation or density.
The most common signs of breast cancer seen on mammography are the following:
1. A cluster of microcalcifications
2. A mass seen as an area of increased radiodensity
3. An area of architectural distortion in the breast parenchyma
4. Skin thickening or edema
5. Rarely, asymmetry alone
These “common signs” can also be seen with benign lesions, leading to a false-positive mammographic rate of 15–20% (11). For example, clusters of microcalcifications are also seen with benign processes such as hyperplasia, adenosis, fibroadenomas, and ductal papillomas. However, for accurate diagnosis, a stereotactic core or excisional biopsy may be required.
Mammography misses approximately 10–15% of cancers. It has a low sensitivity for the detection of infiltrating lobular cancers, and cancers in young women with dense breast parenchyma and little fat (12). If there are suspicious findings on clinical examination, biopsy of the breast must be performed regardless of the mammographic findings (13).
Digital Mammography
Digital mammography (DM) was developed to address some of the limitations of film mammography (FM) (14). Its advantages include speed and higher contrast resolution, which in theory is better for detecting densities and masses in dense tissue. The image contrast can be manipulated to aid in the evaluation of dense breasts, which have low contrast. The advantage of FM is spatial resolution, which is better for detecting calcifications. DM also delivers a smaller dose of radiation than FM, but the differences are of minor significance.
Earlier trials did not demonstrate a significant difference in accuracy between digital and conventional mammography, but were limited by sample size. The Digital Mammographic Imaging Screening Trial (DMIST) involved 49,500 female volunteers who underwent both conventional and digital mammography. Subset analyses indicated that in women under age 50 and in women with dense breasts, DM may be more accurate in detecting breast cancer (15). Because digital images can be stored electronically, most facilities have transitioned to DM. Even though DM may be of benefit in certain subgroups of women, women should not forego annual screening mammograms if DM is not available to them.
Digital Breast Tomosynthesis
Digital breast tomosynthesis (DBT) is a relatively new technology derived from DM. In DBT, a series of cross-sectional images (much like a CT scan) is obtained of the compressed breast, then mathematical algorithms are used to render a 3D mammogram of the breast. This may reduce the effect of tissue overlap and breast density (16). In the United States, approximately 50% of women who undergo screening mammography are categorized as having either “extremely dense” or “heterogeneously dense” breast tissue. Nine states, including California, have enacted legislation requiring written notification of breast density be given to women with mammographically dense breasts, so that they may discuss supplemental screening techniques with their physicians (17).
Early studies suggest that the addition of DBT to screening DM may improve breast cancer detection. The Screening with Tomosynthesis OR standard Mammography (STORM) trial prospectively compared conventional DM and DM with the addition of DBT performed in 7,292 asymptomatic women aged 48 and older at a single institution. Each participant’s DM and DM plus DBT were read independently by a panel of radiologists. A total of 59 cancers were identified in this cohort: 39 were detected on DM and DM plus DBT; an additional 20 cancers were detected only by DM plus DBT. The incremental increase in cancer detection with the addition of DBT to DM was 2.7 cancers per 1,000 screens and the false-positive recall rate was 141 with DM only versus 73 with DM plus DBT (18). RCTs are still needed to prove the benefit and cost-effectiveness of the addition of DBT to screening DM.
Ultrasonography
Breast ultrasonography (US) is a popular imaging technique that is primarily used as an adjunctive tool. The American College of Radiology Imaging Network Trial found that the addition of physician-performed screening breast ultrasonography to annual screening mammography in 2,809 women with mammographically dense breast tissue would detect an additional 1.1 to 7.2 cancers per 1,000 high-risk women, but would substantially increase the number of false-positive biopsies (19).
Current breast cancer screening guidelines state that there is no role for surveying the entire breast using ultrasound; the main use of ultrasound should be to focus on an area identified as abnormal by mammography or clinical examination. Ultrasonography can determine whether a lesion is present, or whether a clinical finding is within the spectrum of normal parenchyma, such as a prominent fat lobule. If a lesion does exist, US can be used to further characterize the finding.
There are clinical indications for the use of ultrasound as the primary imaging modality. These include evaluating palpable findings in young patients (teens and early 20s); pregnant women; and women presenting with erythematous, tender breasts (19), where the diagnostic dilemma is between infection and inflammatory breast cancer. Other uses include the evaluation of axillary lymph nodes, the postoperative examination of fluid collections (seroma, hematoma), as an aid in interventional procedures such as needle aspiration or biopsy, and for the preoperative localization of nonpalpable lesions.
Ultrasonography is 95–100% accurate in differentiating solid masses from cysts. It can aid in the evaluation of a benign-appearing, nonpalpable density identified by mammography. If such a lesion proves to be a simple cyst, no further workup is necessary if the patient is asymptomatic. There are certain criteria that must be met for a cyst to be classified as “simple,” thus falling into the category of BI-RADS 2: Benign finding. These criteria are (i) anechoic; (ii) well-circumscribed round or oval mass with posterior enhancement; and (iii) thin bilateral edge shadows (20).
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) produces detailed cross-sectional images of tissues and structures utilizing magnetic fields. The use of screening and diagnostic MRI is gaining more recognition as an adjunct to mammography in specific clinical scenarios. MRI has associated false-negative rates depending on the specific clinical application, and its specificity is significantly lower than that of mammography. This is caused by the enhancement of benign lesions such as fat necrosis, fibroadenomas, and fibrocystic changes (21), and results in an increased recall and biopsy rate.
Table 16.2 Recommendations for Breast MRI Screening as Adjunct to Mammography

As the technology of MRI has become more sophisticated with dedicated breast MRI coils, contrast agents, and protocols (21), breast MRI is increasingly being used in the clinical setting. MRI is a useful tool in the setting of equivocal mammographic or ultrasonic findings, particularly in patients with dense breasts, postlumpectomy scarring, a strong suspicion of infiltrating lobular carcinoma (ILC), scattered calcifications suggestive of extensive ductal carcinoma in situ (DCIS) or extensive intraductal cancer, bloody nipple discharge, or silicone implants (12).
In women who have an occult primary breast cancer presenting as axillary adenopathy, mammography is limited in its ability to identify the primary breast cancer. There is evidence that MRI can identify occult breast lesions, allowing for more accurate staging and the possibility of breast-conserving surgery (22,23). Clinical studies have shown that MRI can be beneficial in monitoring the response to neoadjuvant chemotherapy (24), in determining the extent of disease, and for contralateral breast cancer screening (25). MRI is also used as an adjunct to mammography in screening high-risk women, including those with BRCA gene mutations and those with an estimated lifetime risk of breast cancer greater than 20%. See Table 16.2 for American Cancer Society MRI screening guidelines (26).
Benign Breast Conditions
Fibrocystic Change
Fibrocystic disease is one of the most common breast problems seen in clinical practice. The term “disease” is misleading and “change” or “condition” is a preferable, more accurate description. Fibrocystic change/condition constitutes a spectrum of clinical signs, symptoms, and histologic features. In a woman with fibrocystic change symptoms, an important part of the evaluation is to exclude malignancy, because the diagnosis of fibrocystic change is otherwise of little clinical significance (27).
Clinical Presentation
Symptoms and Signs
Fibrocystic change is thought to be hormonally related because it appears primarily in women between the ages of 30 and 50 years, subsides after menopause, and can fluctuate with menstrual cycles. Women may present with multiple, tender, palpable masses; usually the breasts are most tender and the masses largest just before the menses, with signs and symptoms abating after menstruation. It is estimated that 60% of women have clinical findings compatible with the diagnosis of fibrocystic change (27). Fibrocystic change involves changes in both the stromal and glandular tissues.
Evaluation
Malignancy must be excluded, but often on physical examination, the breasts are diffusely nodular, with areas of increased density, mainly in the upper, outer quadrants, with no dominant masses. A mammogram should be performed, with possible ultrasound if there is concern about the presence of a mass clinically. A repeat physical examination after the next menstrual cycle is often valuable to document resolution of questionable areas. In the case of a dominant mass, a breast biopsy, preferably needle biopsy or aspiration cytology, is recommended to rule out malignancy. Large cysts may be aspirated if symptomatic.
Any clear, watery, straw-colored, or greenish nipple discharge should be tested for blood by means of a standard guaiac or Hemoccult test. If the discharge is from multiple ducts, bilateral and nonbloody, it is most likely benign. Copious discharge may be a sign of malignancy.
Pathology
The histologic features of fibrocystic change are variable (27). Changes such as fibrosis, cyst formation, hyperplasia (overgrowth of the cells that line the ducts), adenosis (enlargement of breast lobules), and sclerosing adenosis can be seen microscopically. One or more of these histologic features can be found in 50–60% of asymptomatic women (27). In the case of epithelial hyperplasia, it is important to distinguish between usual and atypical hyperplasia. Atypical hyperplasia is associated with an increased risk of future breast cancer.
Cancer and Fibrocystic Change
The conclusion that there is an association between fibrocystic change and cancer was originally drawn because fibrocystic change and malignancy were commonly found together in the same breast (28). However, because 50–60% of women have fibrocystic change and one in every eight women has breast cancer during her lifetime, it is not surprising that these two entities frequently coexist.
Not all women with fibrocystic change are at an increased risk for cancer. Those with histologic features such as fibrosis, cysts, apocrine metaplasia, and mild hyperplasia are at no increased risk (29,30). Histologic features such as sclerosing adenosis and solid or papillary hyperplasia increase the risk slightly (1.5 to 2 times). Women who have atypical hyperplasia, either lobular or ductal, have at least five times the average risk.
Benign Tumors
Intraductal Papilloma
An intraductal papilloma is a benign lesion that can cause serous, serosanguinous, or bloody nipple discharge (31). It is the most common etiology of bloody nipple discharge without an associated mass. True polyps of epithelial-lined breast ducts, intraductal papillomas are often solitary and found in the subareolar location, arising in a major duct close to the nipple. Intraductal papillomas are most frequently observed in women aged 30 to 50 years and typically are not palpable because they are rarely larger than 5 mm. Compression of the breast close to the nipple in the affected quadrant often produces the discharge.
Because malignancies may present with bloody nipple discharge, mammography should be performed to rule out other abnormalities, and biopsy is usually necessary. Fiberoptic ductoscopy is an endoscopic technique that has been developed over the past 15 years for evaluating bloody nipple discharge; it allows direct visualization of the ductal system through a nipple orifice. For diagnosis of nipple discharge, fiberoptic ductoscopy has demonstrated an 88% sensitivity, 77% specificity, 83% positive predictive value, and 82% negative predictive value (32). The technique is not widely used in clinical practice because of limited expertise, high cost, and poor reproducibility.
In addition to ductoscopy, ductal lavage is another screening procedure for women with nipple discharge. Ductal lavage involves irrigation of the duct with saline and cytologic evaluation of the irrigant. Cytologic analysis of ductal lavage alone has a positive predictive value of 72% and a negative predictive value of 50%, but when combined with fiberoptic ductoscopy, the positive predictive value increases to 86% and the negative predictive value increases to 87% (32). There are limitations to the utilization of this technique including patient discomfort, low cytologic yield, and limited accuracy.
Treatment
Local excision of the draining duct is the treatment of choice. This can be performed using local anesthesia through a circumareolar incision by reflecting the nipple away from the breast tissue. A lacrimal probe can be used to assist in locating the offending duct. If it is not possible to identify the duct, total ductal excision of the subareolar duct can be performed through the same incision. The subsequent risk of invasive breast cancer is increased with the presence of atypical hyperplasia in a papilloma, with the risk similar to that of proliferative disease with atypia (33). Papillomas may have malignant epithelium either in situ or invasive (34).
Papillomatosis, defined as a minimum of five papillomas within an excised segment of breast tissue, is benign. Papillomatosis may present as nipple discharge, imaging abnormality, or even a palpable mass. Surgical excision alone is sufficient (32). There is an increased risk of cancer in patients with papillomatosis.
Fibroadenoma
Fibroadenomas are the most common benign breast mass in women (27). They are noncancerous growths composed of epithelial and stromal elements. They rarely occur after menopause, but occasionally calcified fibroadenomas are found in postmenopausal women. It is believed that they are influenced by estrogenic stimulation (27).
Symptoms and Signs
Clinically, a young patient usually notices a mass while showering or dressing. Most masses are 1 to 3 cm in diameter, but they can grow to an extremely large size (i.e., the giant fibroadenoma). On physical examination, they are firm, rubbery, smooth, and freely mobile. Fibroadenomas may be multiple. On mammography, they may appear as circumscribed, oval, or round masses. Occasionally, coarse calcifications can be seen within a fibroadenoma. On ultrasonography, they characteristically appear as circumscribed, homogeneous, hypoechoic oval masses with occasional lobulations, and are wider rather than tall. On MRI, they typically appear as smooth masses with high signal intensity on T2-weighted images.
Although the risk of cancer in a fibroadenoma is extremely low, some clinicians choose to biopsy (with fine-needle aspiration [FNA], core needle, or excisional biopsy) any solid mass in patients older than 30 years for definitive diagnosis. A lesion that is benign on ultrasonography and mammography is benign more than 99% of the time. Some clinicians will omit biopsies in younger women with lesions characteristic of fibroadenomas and will follow these patients with serial ultrasonography (27).
Complex fibroadenomas are fibroadenomas that contain cysts, sclerosing adenosis, papillary apocrine changes, or epithelial calcifications. In a clinical follow-up study by Dupont et al. (35), complex fibroadenomas were shown to be associated with a slightly increased risk of breast cancer. Patients who have simple fibroadenomas without complex histologic features are at no increased risk for development of invasive cancer.
Treatment
Although complete excision under local anesthesia can treat the lesion and confirm the absence of malignancy, excision is not often necessary. A fibroadenoma diagnosed by clinical examination, imaging and needle biopsy may be followed if the lesion remains stable. If a fibroadenoma increases in size, it should be excised. Excision is recommended for fibroadenomas that are greater than 2 or 3 cm to rule out phyllodes tumor. Fibroadenomas may diminish in size or even totally resolve, particularly in younger women; therefore excision can be avoided (36).
Benign Phyllodes Tumor
Phyllodes tumors are fibroepithelial breast tumors characterized by stromal overgrowth and hypercellularity combined with an epithelial component, grossly forming a leaf-like structure. Clinically, phyllodes tumors tend to occur in women aged 35 to 55 years, and comprise less than 1% of breast tumors (37). These lesions usually appear as isolated masses that are difficult to distinguish from fibroadenomas (38). Size is not a diagnostic criterion, although phyllodes tumors tend to be larger than fibroadenomas, often with a history of rapid growth. Both appear as well circumscribed, oval or lobulated masses, with round borders on mammography and ultrasonography. There are no good clinical or radiographic criteria by which to distinguish a phyllodes tumor from a fibroadenoma (37,38).
Pathology
Phyllodes tumors are classified as benign, borderline, or malignant based on the histologic criteria first described in 1978 (39). These histologic criteria are based on features such as the number of mitoses, pushing or infiltrative tumor margins, degree of stromal overgrowth, and degree of stromal cellular atypia. These are all used in combination to distinguish between the benign and malignant spectrum (40). Even with histologic criteria, definitive distinction between fibroadenoma, benign phyllodes tumor, and malignant phyllodes tumor can be very difficult, and the correlation of histologic grade to clinical behavior and outcome has been challenging. Even “benign” phyllodes tumors tend to recur locally, particularly if the tumor is simply enucleated. Malignant phyllodes tumors have a higher local recurrence rate and can metastasize, usually to the lungs (40,41); for this reason, these tumors were originally called cystosarcoma phyllodes. Axillary lymph node metastases are extremely unusual despite the large size of some phyllodes tumors that are classified as “malignant.”
Treatment
Because of the high propensity for local recurrence, the treatment of phyllodes tumors should consist of a wide, local excision (40–42). Large tumors not amenable to breast conservation and malignant tumors with particularly infiltrative margins may require total mastectomy without axillary node dissection; however, mastectomy should be avoided whenever possible. These malignant tumors are rarely multicentric and rarely metastasize to lymph nodes. Typically, a phyllodes tumor is discovered by histologic examination after a patient has undergone an excisional biopsy of a mass believed to be a fibroadenoma. When the pathologic diagnosis is phyllodes tumor, a complete re-excision of the area should be undertaken so that the prior biopsy site and any residual tumor are excised. There is no role for adjuvant therapy, either radiation therapy or chemotherapy.
Breast Cancer
Breast cancer is the most common cancer in women under the age of 60, and is second only to lung cancer as the leading cause of cancer deaths in women. It accounts for 29% of all new cancers in women. In 2013 in the United States, an estimated 232,340 new cases of invasive breast cancer will be diagnosed in women, with approximately 39,620 deaths (43). The overall lifetime risk for development of breast cancer in women in the United States is one in eight or 12.38% (43).
During the past 50 years, there has been a significant increase in the incidence of breast cancer in the United States. This correlates with the increased use of screening mammography (44). Breast cancer incidence rates increased after 1980, but decreased by 3.5% per year from 2001 to 2004 probably as a result of declining use of hormonal replacement therapy (HRT) by postmenopausal women, and delays in diagnosis caused by a decrease in mammographic utilization (45).
The mortality rate has dropped slightly, thought to result in part from mammographic screening and improvements in systemic therapy (3). Screening mammography has also resulted in a decrease in the size of breast cancer at diagnosis, with close to one-third of cancers being 1 cm or less in diameter (46). Not surprisingly, nodal involvement has decreased and the proportion of DCIS cases has increased. It is predicted that in the next decade, these trends will continue.
Predisposing Factors
One of the fundamental steps in determining a patient’s risk for breast cancer is to obtain a detailed history. This allows the physician to plan preventive and diagnostic strategies, as well as to educate the patient about breast cancer. Intrinsic and extrinsic factors contribute to increasing a woman’s risk. Intrinsic characteristics include genetic and familial elements, age, endogenous hormonal exposures, and benign breast lesions with high-risk histologies. Extrinsic characteristics include environmental exposures, diet, and exogenous hormonal exposures.
Age
Breast cancer is rare before the age of 25 years, accounting for less than 1% of all cases. After the age of 30 years, there is a sharp increase in the incidence, with a small plateau between the ages of 45 and 50 years, consistent with the involvement of hormonal factors (47). The risk of breast cancer in the next decade of life is 0.44% for a 30-year-old woman, 1.45% for a 40-year old, 2.31% for a 50-year old, 3.49% for a 60-year old, and 3.84% for a 70-year old (48).
Prior History of Breast Cancer
One of the strongest single risk factors for the development of breast cancer is the previous diagnosis of a contralateral breast cancer. The risk of subsequent contralateral breast cancer in a patient with unilateral breast cancer has been reported to range from 0.5–1% per year (49–52). Young age (49,50,52) and lobular histology (50–52) have been associated with a greater likelihood of contralateral breast cancer and adjuvant chemotherapy with a decreased likelihood (51,52). In patients diagnosed with unilateral breast cancer, breast MRI has been shown to detect clinically and mammographically occult contralateral cancers in 3.1% of cases at the time of diagnosis (25).
Family History
A family history of breast cancer increases a patient’s overall relative risk (53). However, the risk is not significantly increased for women with first-degree relatives (mother, sister) with postmenopausal breast cancers, whereas women whose mothers or sisters had bilateral premenopausal breast cancer have a high likelihood of acquiring the disease. If the patient’s mother or sister had unilateral premenopausal breast cancer, the likelihood of the patient developing breast cancer is approximately 30%. If a woman has several first-degree relatives with breast cancer, the risk increases.
Inherited Syndromes of Breast Cancer
The majority of breast cancers occur sporadically, with only approximately 5–10% attributable to a breast cancer susceptibility gene (54). Two breast cancer susceptibility genes, BRCA1 mapped to chromosome 17q21, and BRCA2 on chromosome 13q12 to 13, are high penetrance tumor suppressor genes inherited in an autosomal-dominant fashion (55,56). Mutations in these genes account for only about 15% of all familial breast cancers, suggesting that other breast cancer susceptibility genes exist (57).
The estimated lifetime risk of breast cancer for women who have BRCA1 mutations ranges from 36% to as high as 87%, with a pooled estimate of 70% (56–59). The cumulative risk of ovarian cancer in BRCA1 carriers has been reported to be between 27% and 45% (58–60). For BRCA2 mutation carriers, lifetime estimated breast cancer risk is 60% (range 45–84%) and lifetime estimated ovarian cancer risk is 20% (59). BRCA2 mutations are also associated with a 6% lifetime risk of male breast cancer. Furthermore, 10–20% of BRCA1–2 mutation carriers treated with breast conservation at initial diagnosis will develop contralateral breast cancer within 10 years. The likelihood of contralateral breast cancer increases with young age at diagnosis and number of first-degree relatives with breast cancer (59,61). Breast cancers that occur in women with BRCA1 mutations are mostly estrogen-receptor (ER) negative (up to 90%) and of high nuclear grade (59). BRCA mutations are also associated with increased risk for the development of additional cancers such as colon, pancreatic, uterine, and cervical (59,62).
The prevalence of BRCA1 and BRCA2 in the general population is unknown but is estimated to be less than 0.12% and 0.044%, respectively (59). In Ashkenazi Jewish women without breast cancer, the prevalence of these mutations is as high as 2% (63). In women diagnosed with breast cancer before the age of 32, the incidence of BRCA1 or BRCA2 mutations is approximately 12% and in Ashkenazi women diagnosed with breast cancer before age 40, the incidence is 20% (64,65). There are a large number of potential mutations and other genetic modifiers, and the penetrance of the various mutations is highly variable. It is being studied by the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) (66).
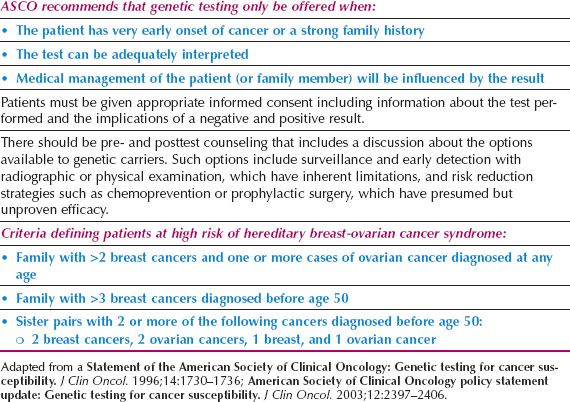
There are other hereditary syndromes associated with breast cancer with mostly autosomal dominant transmission, such as Li–Fraumeni, Cowden disease, Muir–Torre, a variant of hereditary nonpolyposis colon cancer (HNPCC), ataxia–telangiectasia (autosomal recessive), and Peutz–Jeghers syndromes. Each syndrome is associated with an abnormal gene responsible for producing a recognizable phenotype. In clinical practice, these syndromes contribute to only a small fraction of hereditary breast cancers. The American Society of Clinical Oncology has specific guidelines for genetic testing for cancer susceptibility that are summarized in Table 16.3 (67).
The American Society of Breast Surgeons (ASBS) issued a statement in 2006 defining patients at high risk for breast cancer. Persons in this high-risk population were those with early onset breast cancer (before age 50), two primary breast cancers, a family history of early onset breast cancer, a previously identified BRCA1/BRCA2 mutation in the family, a personal or family history of ovarian cancer, or Ashkenazi Jewish heritage (https://www.breastsurgeons.org/statements/PDF_Statements/BRCA_Testing.pdf). Patients included in this group have a 10% or greater risk of harboring a BRCA1 or BRCA2 mutation, which is the traditional cutoff for testing.
Reproductive and Hormonal Factors
It is thought that lifetime exposure to endogenous estrogen plays a promotional role in the development of breast cancer (68–70). Women with breast cancer begin menses at a younger median age (70), and the longer a woman’s reproductive phase, the higher the risk of breast cancer (68). There is no clear association between the risk of breast cancer and duration of menses or menstrual irregularity. Studies of the effect of lactation have been inconclusive, but childbearing definitely has an effect on breast cancer risk (69). Women who have never been pregnant have an increased risk compared to women who are parous, and late age at first birth also increases the risk of breast cancer (69).
Table 16.3 American Society of Clinical Oncology: Genetic Testing for Cancer Susceptibility Guidelines
Meta-analyses of prospective cohort studies have demonstrated a small, increased risk of breast cancer for women who have ever taken oral contraceptives (odds ratio 1.08; 95% confidence interval: 0.99 to 1.17). Furthermore, every 10 years of oral contraceptive use is associated with a 14% increase in breast cancer risk (71). Other analyses suggest that the risk declines with cessation of oral contraceptive use and, after 10 or more years from cessation, there appears to be no excess risk. Breast cancers in women who had used oral contraceptives tended to be less advanced clinically and localized to the breast (72).
The association between breast cancer and HRT in postmenopausal women has been investigated in two large randomized trials. In the Women’s Health Initiative (WHI) study, investigators demonstrated that women randomized to combination estrogen plus progesterone HRT, had a significantly increased incidence of breast cancer, stroke, and pulmonary embolus compared with those randomized to a placebo (73). HRT however, significantly decreased the incidence of colorectal cancer and femoral neck fractures. The WHI trial was stopped prematurely after it became apparent that the risks outweighed the benefits. The risk of breast cancer has been shown to be greater for combination versus estrogen-only HRT. However, women receiving estrogen-only HRT after hysterectomy in this study were at an increased risk for stroke (74). The Million Women Study in the United Kingdom recruited 1,084,110 women and also demonstrated that current use of HRT was associated with an increased risk of breast cancer (75).
Soon after the report of the WHI, the use of HRT in the United States decreased by 38%, with approximately 20 million fewer prescriptions written in 2003 than 2002 (76). Analysis of data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registries indicates that the age-adjusted incidence rate of breast cancer in women fell by 6.7% in 2003. This decrease in breast cancer incidence seems to be temporally related to the drop in the use of HRT by postmenopausal women. This decrease occurred primarily in non-Hispanic white women who were the primary users of HRT (77). Follow-up data from the WHI study has demonstrated that the risk of breast cancer associated with combination HRT began to decrease toward baseline risk levels at 2 years from HRT cessation (78).
In counseling women with complaints of postmenopausal symptoms, the decision to initiate combination HRT must be individualized. For some patients, the improved quality of life and protection against fractures outweigh the potential risks. The individual risk of breast cancer in a particular postmenopausal patient should be considered before initiating HRT. If HRT is to be prescribed, low dosage formulations should be used with the understanding that the risk for harm will likely increase with prolonged use, and the survival benefit will diminish over time. For women at high risk for fractures, bisphosphonates are a suitable alternative. Any postmenopausal woman on HRT should be aware of the increased risks of breast cancer, and should be counseled to be particularly diligent regarding breast cancer awareness and screening.
Diet, Obesity, and Exercise
Regarding diet and breast cancer, the majority of epidemiologic studies are case-control studies and cohort studies. Few RCTs have been conducted and are problematic because the randomized dietary plan has to be adhered to for a many years. The association between breast cancer risk and dietary fat intake, for example, has been inconsistent. One large meta-analysis of 11 prospective cohort studies found no evidence of a positive association between fat intake and risk of breast cancer when those in the highest quintile of total fat intake were compared with those in the lowest quintile (79). Similarly, a large RCT involving 40 US centers from the WHI study followed 48,835 postmenopausal women for 8 years who were randomly assigned to a dietary modification group or a control group (80). The dietary modifications included five servings of fruits and vegetables, six servings of grains, and total fat to 20% of energy requirements daily. In the group of women who had a low-fat diet, there was no statistically significant reduction in invasive breast cancer risk. The results were confounded by relatively few women meeting the low-fat dietary target (only 14.4% at year 6). Future studies were encouraged because it may take years for the benefits of a low-fat diet to be manifested.
One large prospective study in the United States of 90,655 premenopausal women found no association between fiber intake and the risk of breast cancer, whereas a study in the United Kingdom of 35,792 women demonstrated a reduced risk of breast cancer for premenopausal women who consumed more than 30 g of fiber per day as compared to those who consumed less than 20 g/d (81,82). Studies on soy intake have also yielded mixed results. Historically, breast cancer incidence has been lower in Asia where dietary soy intake is significantly higher than in a typical American diet. This may be counterintuitive because soy protein is broken down into estrogenic compounds. A recent meta-analysis of four studies of breast cancer recurrence and 14 studies of breast cancer incidence demonstrated that soy consumption was inversely associated with breast cancer incidence (RR = 0.89, 95% CI: 0.79 to 0.99). However, the protective effect of soy was only observed among studies conducted in Asian populations (RR = 0.76, 95% CI: 0.65 to 0.86), not in Western populations (RR = 0.97, 95% CI: 0.87 to 1.06). Soy intake was also inversely associated with risk of breast cancer recurrence (RR = 0.84, 95% CI: 0.70 to 0.99) (83). Studies of natural antioxidants, the dietary carotenoids, and vitamins A, C, E, and D have also been conflicting. While early observational studies suggested an association between low levels of 25-hydroxy vitamin D and breast cancer, pooled analyses of subsequent observational studies have not demonstrated a reduction in breast cancer risk associated with vitamin D supplementation (84).
The evidence for obesity as a risk factor for breast cancer development is much greater. Obesity, as defined by a body mass index (BMI) greater than or equal to 30 kg/m2, is a rapidly growing problem that has been associated with increased risk of cancer development. Increased adiposity has been associated with increased levels of proinflammatory cytokines and adipose tissue-derived cytokines leptin and adiponectin, which can initiate a number of molecular pathways associated with cancer development (85). The Million Women Study in the United Kingdom, for example, demonstrated a linear correlation between increasing BMI and increasing risk of postmenopausal breast cancer incidence and mortality (86). This increased risk has not been demonstrated for premenopausal breast cancer (86,87).
Exercise has been shown to reduce the risk of postmenopausal breast cancer (88). A recent study of 64,777 women from the Nurses’ Health Study Cohort II evaluated premenopausal exercise habits and showed that the most active women (running 3.25 hours or walking 13 hours per week) had a 23% reduction in the risk for premenopausal breast cancer (89). A large systematic review supports the decreased risk of breast cancer with exercise: 62 studies were evaluated with the conclusion that increased physical activity led to an overall risk reduction for breast cancer of 25%, with a dose-dependent effect noted in 28 of the studies (90). This reduction in risk was seen in both premenopausal and postmenopausal women.
The metabolic effect of exercise and its impact on breast cancer risk biomarkers are being investigated in a randomized, multicenter SHAPE-2 trial of 243 sedentary, postmenopausal women with BMI 25 to 35 kg/m2 (overweight or obese). Participants have been randomized to one of three groups: Diet alone, exercise alone, or diet plus exercise. The results of the SHAPE-2 trial may lead to more specific lifestyle guidelines for breast cancer prevention (91).
Observational studies have shown that moderate exercise may reduce the risk of breast cancer recurrence and mortality and may be protective against second breast cancers (92). The Health, Eating, Activity, and Lifestyle (HEAL) study of 688 women with early stage breast cancer found that women who expended the equivalent of brisk walking 2 to 3 hours per week had a hazard ratio for death of 0.33 compared with sedentary women (93). Likewise, the WHI study demonstrated lower breast cancer-specific mortality for women who engaged in 3 hours per week of fast walking after their cancer diagnosis, even if they were sedentary previously (HR 0.61, 95% CI 0.44 to 0.87, p = 0.01) (94).
Alcohol
Regular alcohol consumption of as little as half a drink (1 drink = 10 g) per day has been linked to an increased risk of breast cancer. This increase is seen irrespective of the type of alcoholic beverage consumed. The Million Women Study in the United Kingdom found that consumption of an average of 1 drink per day in middle-aged women was associated with a 12% increase in breast cancer risk (95). The Nurses Health Study in the United States found that after 2.4 million person-years of follow-up, 7,690 cases of invasive breast cancer were diagnosed among the 105,986 original participants, and that consumption of as little as 0.5 to 1 drink per week was associated with an increased breast cancer risk (RR 1.15, CI, 1.06 to 1.24). Furthermore, alcohol intake early or late in adult life is an independent risk factor for breast cancer (96).
Radiation Exposure
Prior radiation exposure to the thoracic area, especially during adolescence or early adulthood (when breasts are developing), has been shown to increase the risk of breast cancer. In Japan, female atomic bomb survivors have been noted to have an increased risk of breast cancer, and the risk was greatest for women who were exposed as children (97). Multiple studies, including the Childhood Cancer Survivor Study, have demonstrated an increased risk for women who have undergone treatment for Hodgkin disease. In this study, the 30-year incidence of invasive breast cancer among 878 female survivors of Hodgkin disease was 18% (98). The increased risk is primarily age related, with the highest risk associated with treatment at ages 10 to 20 years. This increased risk warrants high-risk surveillance with annual screening breast MRI, in addition to annual mammography (26). Lower doses of therapeutic radiation, such as with fluoroscopy previously used to treat tuberculosis, have also been associated with an increased risk (99).
Diagnosis
The majority of breast tumors are found in the upper, outer quadrant, where there is more breast tissue. Breast cancer is often discovered by the patient. The findings on mammography may suggest that a palpable lesion is malignant. On the other hand, screening mammography may detect an abnormality without a palpable tumor. Rarely, the patient may present with an axillary mass and no obvious carcinoma in the breast.
A breast mass in a woman of any age must be approached as a possible carcinoma. At times, the only clue to an underlying malignancy may be a subtle finding of an area of thickening amid normal nodularity. Obvious clues to malignancy include nipple retraction, skin dimpling, involved nodes, or ulceration, but these are late signs and are not common at presentation.
After obtaining the history and physical examination, the “triple test” should be utilized to evaluate a palpable mass. Triple test refers to the combination of physical examination, breast imaging (usually mammogram ± ultrasound) and pathologic diagnosis employing cytology by means of FNA, or histology by means of a core-needle biopsy (CNB) or excisional biopsy. The diagnostic accuracy is approximately 100% when all three are concordant (100,101). Indications for an excisional biopsy include cytologic or histologic atypia on needle biopsy, any discordance between any of the three modalities, or the patient’s desire to eliminate a source for concern. Algorithms for the evaluation of breast masses in premenopausal and postmenopausal women are presented in Figs. 16.3 and 16.4. The ACS reports that 80% of breast biopsies are benign (http://www.cancer.org).

Figure 16.3 Schematic evaluation of breast masses in premenopausal women. 1Complex mass—a cystic mass with a solid component that may be malignant. 2Complicated cyst—usually multiple simple cysts or cysts with septations.

Figure 16.4 Schematic evaluation of breast masses in postmenopausal women.
Fine-Needle Aspiration Cytologic Testing
FNA is performed with a 20- or 22-gauge needle, and has a high diagnostic accuracy, with a 10–15% false-negative rate. FNA has a rare but persistent false-positive rate, often in association with epithelial proliferative lesions such as ductal or lobular hyperplasia (102,103). A negative FNA cytologic diagnosis that is discordant with CBE and imaging must be followed with either core needle biopsy or excisional biopsy. FNA is limited by the availability of a skilled cytopathologist and the inability of the technique to distinguish between noninvasive and invasive breast cancer and between ductal and lobular subtypes of invasive breast cancer (104).
Biopsy
Core-Needle Biopsy
CNB is a less invasive procedure than open biopsy. It utilizes an 8- to 14-gauge needle to obtain specimens. Abnormal architecture and invasion can be identified in these tissue samples, unlike FNA (105). Specific tumor markers such as estrogen receptor (ER), progesterone receptor (PR), or HER2/neu can be performed on core biopsy-diagnosed breast cancers (106).
Indications for CNB are the same as for open biopsy and CNB may be performed handheld for palpable masses. Suspicious nonpalpable lesions can be biopsied using stereotactic CNB for lesions seen on mammography, or with ultrasonic or MRI guidance for lesions seen on ultrasound or MRI, respectively. CNB using ultrasonic guidance is simpler and less expensive than stereotactic CNB, and does not require special equipment (105).
A number of studies have shown sensitivity and specificity ranging from of 85–100% for image-guided CNB. The sensitivity and negative predictive values of CNB are less for mammographic calcifications (0.84 and 0.94, respectively) than for the diagnosis of masses (0.96 and 0.99, respectively) (104,105). When CNB is used to sample microcalcifications, radiography of the specimen must be performed to ensure the calcifications are present in the tissue removed. A microclip is usually placed after CNB to indicate the biopsied area and a postbiopsy mammogram is obtained. CNB usually does not result in architectural distortion, which may alter the interpretation of future mammograms.
Certain technical issues may not allow CNB to be employed, such as very superficial or posterior lesions. Mammographic lesions in a very small breast are usually not amenable to stereotactic core biopsy, and breast implants can make CNB more challenging. Depending on the location of the lesion in relation to the implant, open biopsy is sometimes recommended to avoid implant rupture. Vacuum-assisted devices with CNB allow for multiple samples to be obtained without withdrawing and reinserting the needle.
Open Biopsy
A minority of patients requires surgical biopsy if the lesion is not amenable to CNB, the sample is inadequate, or the pathologic results are equivocal. Likewise, follow-up open biopsy is indicated if benign diagnoses of atypical ductal hyperplasia (ADH), flat epithelial atypia, atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), intraductal papilloma, or radial scar are made after CNB, because of the coexistence of malignancy in up to 20–30% of such cases (107).
Image-Guided Open-Biopsy
Nonpalpable lesions detected by a mammogram, ultrasound, or MRI that are not amenable to CNB require open biopsy after preoperative localization by imaging. This requires the collaborative effort of the surgeon and the radiologist and entails the placement of a needle or specialized wire into the suspicious area. To further assist in localization, many radiologists also inject a biologic dye. The surgeon reviews the films and localizes the abnormality with respect to the tip of the wire or needle and plans the operation accordingly.
Open biopsy can usually be performed in the outpatient setting with the aid of intravenous (IV) sedation and local anesthesia. The following steps are undertaken:
1. IV sedation is used to ease anxiety. Local anesthesia is used to infiltrate the skin and subcutaneous tissue surrounding the mass or wire-localized abnormality.
2. An incision is often made directly over the mass or localized area, at the site of the wire insertion, or periareolar. The incision should be placed cosmetically so that a partial mastectomy can be performed through the same incision. If the tumor is far from the areola, circumareolar incisions are best avoided.
3. After the skin and underlying tissues have been incised, the mass or localized area can be gently grasped with a stay suture or with Allis forceps and delivered into the operative field.
4. Whenever possible, the lesion should be totally excised. An incisional biopsy may be performed for large masses, but a frozen section should be done to confirm that malignant tissue has been obtained. It is important not to remove too much additional breast tissue, because the main purpose of the procedure is to achieve a tissue diagnosis.
5. In the case of abnormalities localized by mammography, a postexcisional mammogram of the surgical specimen should be performed to be certain that the lesion in question has been excised.
6. After adequate hemostasis has been achieved, the wound should be irrigated, the specimen confirmed to contain the lesion by mammography, and the incision closed. The most superficial subcutaneous fat is reapproximated with fine, absorbable sutures, usually a 3–0 or 4–0 vicryl. The skin is best closed with an absorbable subcuticular suture, with either Steri-Strips or Dermabond applied to the incision to achieve the most cosmetically pleasing result.
Two-Step Approach
The two-step approach involves the initial biopsy, either by needle or open procedures, followed by subsequent definitive treatment. Women who are diagnosed with breast cancer on biopsy can discuss the various treatment options and seek out additional consultations, if desirable, before undergoing definitive treatment. For most patients, being engaged in the planning of therapy is an important psychological aspect in the healing process.
Pathology and Natural History
Breast cancer constitutes a heterogeneous group of histopathologic lesions. Regardless of the histologic type, most breast cancers arise in the terminal duct lobular unit. The classification of the World Health Organization (WHO) is most widely used for invasive breast cancers, and it recognizes invasive carcinoma as “ductal” and “lobular.” The WHO classification scheme is based on cytologic features and growth patterns of the invasive tumor cells, with the distinction between lobular and intraductal carcinoma based on the histologic appearance rather than the site of origin (108). Breast cancer may be either invasive (infiltrating lobular carcinoma, ILC), which indicates invasion into the breast stroma with the potential for lymph node and distant metastases, or in situ (DCIS or LCIS), which indicates the inability to spread to other sites.
The most common histologic diagnosis of invasive breast carcinoma is infiltrating ductal carcinoma (IDC). This histologic type accounts for 60–75% of breast cancers in the United States (109). This diagnosis is actually a diagnosis by default, because this tumor type is defined as a type of cancer that does not fall into any of the other categories of invasive mammary carcinoma (108), as recognized by defined histologic features (mucinous, tubular, medullary). Mammographically, it is often characterized by a stellate appearance with microcalcifications. The classic macroscopic appearance of infiltrating ductal carcinoma is a firm, often rock-hard mass that has gritty, chalky streaks within the substance of the tumor. This consistency is caused by the fibrotic response of the surrounding stroma. Microscopically, the appearance is highly heterogeneous with regard to growth pattern, cytologic features, mitotic activity, and extent of in situ component.
The second most common histologic diagnosis is infiltrating lobular carcinoma (ILC), which comprises 5–15% of cases (109). It may present as a mammographic abnormality or palpable mass, as with invasive ductal carcinoma, but the extent of disease may be underestimated by the physical or radiographic findings. Invasive lobular carcinoma often presents as multifocal disease in the ipsilateral breast, with coexistent LCIS in approximately 5–15% of cases (110). The incidence of contralateral breast cancer in ILC patients ranges from 6–47% (109). Macroscopically, some invasive lobular carcinomas may appear as firm, gray-white masses similar to invasive ductal cancers, while others may have only a rubbery consistency of the breast tissue. Microscopically, invasive lobular cancer is characterized by small, uniform neoplastic cells infiltrating the stroma in a single-file pattern, with little or no desmoplastic stromal reaction (108). Other types of invasive breast carcinoma are far less common, and are subtypes of infiltrating ductal carcinoma.
Medullary carcinoma accounts for approximately 2–5% of cases, and is often well circumscribed grossly, with a dense lymphocytic infiltrate microscopically (109). They are usually slow growing, less aggressive cancers. The distant recurrence-free survival was 89% at 14 years in one series, compared with 64% for usual infiltrating ductal carcinomas (111). Tubular carcinoma is a well-differentiated breast cancer with limited metastatic potential and an excellent prognosis, occurring in about 1% of breast cancers (109). Mucinous (colloid) carcinoma accounts for fewer than 5% of all breast cancers, and is also associated with a favorable prognosis (109). Grossly, the tumors are well circumscribed and may have areas that appear mucinous or gelatinous. Microscopically, small clusters or sheets of tumor cells are dispersed in pools of extracellular mucin (108,109).
Papillary carcinoma is used to describe a predominantly noninvasive ductal carcinoma; invasive papillary carcinomas are rare, accounting for approximately 1% of breast cancers (108,109). An extremely rare form of breast cancer is adenoid cystic carcinoma, which is similar histologically to the salivary gland tumor. They often present as a palpable mass with no clinicoradiologic features. These cancers metastasize late and tend to be well differentiated (109,112).
Growth Patterns
The tumor growth rate of a breast cancer varies widely among patients and at different stages of the disease. Many mathematical models have been devised based on the natural history of breast cancer, assumptions of tumor growth rate, the probability of detecting a tumor of a given size, and the rate of clinical surfacing (113). Doubling time of breast cancer has been estimated to range from several weeks for rapidly growing tumors to months for slowly growing ones. Based on seminal studies, the mean doubling time for mammary carcinomas has been estimated to be 5.4 months, with a standard deviation of 4 months (114). To give an example for clinical application, if it is assumed that the doubling time is 100 days, the doubling time is constant (which is not often the case), and the tumor originated from one cell, it would take 8 years to result in a 1-cm tumor (Fig. 16.5) (115). Before the tumor is clinically apparent, tumor cells may be circulating through the body.

Figure 16.5 Growth rate of breast cancer, indicating long preclinical phase. (From Gullino PM. Natural history of breast cancer: Progression from hyperplasia to neoplasia as predicted by angiogenesis. Cancer. 1977;39:2699. Copyright 1977 American Cancer Society. Reprinted by permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree