Abstract
Organization, more than 20 morphologic subtypes of breast cancer have been recognized. By gene expression, invasive breast cancer is classified into at least four major subtypes; luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)/neu-positive, and triple-negative cancers. The biology and the prognosis of these subtypes are vastly different. It is well recognized that genetic profiles of these cancer subtypes are also interlinked with the traditional breast biomarkers. According to the US National Institutes of Health’s Working Group and Biomarkers Consortium, a biomarker is a characteristic that is objectively measured as an indicator of normal biological processes, pathogenic processes, or a pharmacologic response to a therapeutic intervention.
Keywords
biomarker, immunohistochemistry, pathology, hormone receptors
Breast cancer is a heterogeneous disease process. According to the World Health Organization, more than 20 morphologic subtypes of breast cancer have been recognized. By gene expression, invasive breast cancer is classified into at least four major subtypes: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)/neu-positive, and triple-negative cancers. The biology and the prognosis of these subtypes are vastly different. It is well recognized that genetic profiles of these cancer subtypes are also interlinked with the traditional breast biomarkers. According to the US National Institutes of Health’s (NIH’s) Working Group and Biomarkers Consortium, a biomarker is a characteristic that is objectively measured as an indicator of normal biological processes, pathogenic processes, or a pharmacologic response to a therapeutic intervention. For more than 40 years, estrogen receptor (ER) has been recognized to play a crucial central role in the development and progression of breast cancer. This knowledge has been used to develop treatment protocols targeting this biomarker to block the progression and recurrence of breast cancer. With the passage of time, additional biomarkers were discovered. Today ER, progesterone receptor (PR), and HER2/neu are considered important biomarkers that can predict not only the response to treatment but also prognosis and potentially disease recurrence. The reporting of these biomarkers is now almost completely standardized throughout the United States. Other commonly used but less defined biomarkers are Ki67, a proliferation marker, and p53. Currently, there is no universal protocol for reporting. The significance of p53 expression is even less defined; therefore, this biomarker will not be discussed.
Epithelial cadherin (E-cadherin) and p120 that are used to confirm diagnosis of lobular differentiation are not traditionally considered breast biomarkers. Because invasive lobular carcinoma is thought to have a different prognosis independent of the stage, these immunohistochemical markers should also be considered in the category of breast biomarkers.
Estrogen Receptor: Historical Perspective
ER status has long been recognized as an important predictive and prognostic biomarker in breast cancer. Adjuvant hormonal therapy has shown considerable benefits in terms of reduced overall disease recurrence and 15-year mortality from breast cancer in women of all ages with ER-positive tumors (that generally represents more than 75% of all breast tumors). ER assessment by immunohistochemistry (IHC) is now the standard in the management of breast cancer. Like other steroid receptors, ER belongs to the nuclear receptor superfamily. It consists of 553 amino acids forming an N-terminal domain with transcription activation functions, a central DNA-binding domain and a ligand-binding domain at the carboxy terminal. Two isoforms of ER exist: ERα and Erβ, which are encoded by two different genes with no homology in their amino acid sequences. Although the specific roles of ERβ are still being elucidated, ERα is the most studied and clinically measured isoform. The ligand for ER is the female sex hormone 17β-estradiol (E2) that in the physiologic state mediates growth and differentiation of the breast ducts. Estrogen signaling pathway is activated when the hormone diffuses through the cell membrane and binds to nuclear ER, inducing its dimerization. The ligand-receptor complex then promotes downstream gene transcriptions.
The association between estrogen hormone and breast cancer pathogenesis has been known since the late 1800s. Objective evidence of tumor regression with oophorectomy, adrenalectomy, or hypophysectomy has been documented for metastatic and inoperable breast tumors. The initial report on the characterization and measurements of ER was from Jensen and associates in the mid-1960s. A few years later, McGuire and colleagues observed that there was variability in the concentration of ER in primary and metastatic breast cancers and emphasized that an assay for ER must be quantitative. Their studies provided the early insights into this hormone receptor’s utility as a biomarker for breast cancer.
Before the era of IHC, ligand-binding assays were used primarily for receptor quantitation. These biochemical procedures were often cumbersome and involved extraction of receptor proteins by homogenization of fresh-frozen tumor tissue, incubation of the homogenate with radioactive and nonradioactive E2, followed by separation of the bound and unbound hormone. Scatchard plots and standard curves were subsequently used to quantify ER and expressed in femtomoles of ER protein per milligram of cytosol protein. Some of the earlier receptor analytic methods included Sephadex gel filtration, protamine sulfate precipitation, sucrose density gradient ultracentrifugation, and dextran-coated charcoal assay. The latter two methods were commonly used, with dextran-coated charcoal assay preferred because of its ease of use and its accuracy. Although newer methodologies such as enzyme immunoassays and IHC have made ligand-binding assays obsolete, they were the first assays that broadened our understanding of ER and the response to hormonal therapy, helped determine positive and negative cutoff levels for ER, and were even used to validate the newer assays.
The application of IHC for ER gained widespread acceptance in the 1990s with the development of new monoclonal antibodies to ER and different antigen retrieval techniques. IHC offers many advantages over the traditional biochemical assays; importantly, it can be applied on very small amounts of tumor in formalin-fixed, paraffin-embedded tissue as well as frozen tissue. The immunohistochemical stain can be directly applied to a microscopic slide, which permits direct visualization of anti-ER antibodies binding to tumor cells and helps differentiate ER staining of stromal cells, necrotic tumor, and benign parenchyma from the tumor cells. Furthermore, in situ and invasive components and different morphologic subtypes can be selectively evaluated with IHC. Worldwide, the current practice is to use IHC exclusively on paraffin sections to assess ER status of breast cancers.
Progesterone Receptor: Historical Perspective
Like ER, PR status is an independent predictive factor for benefit from adjuvant endocrine therapy and a prognostic indicator for early recurrence in breast cancer. This discovery was preceded by observations that a subset of ER-positive breast cancers failed to respond to hormonal manipulation, indicating that ER presence alone was not a sufficient indicator of hormone dependence in breast cancer. It was shown that ER-positive/PR-positive breast cancers fared better compared with ER-positive/PR-negative tumors to adjuvant treatment. Studies by Horowitz and associates, the same group that extensively studied ER, suggested that the presence of PR might serve as an indicator of the functionality of the estrogen signaling pathway in the breast.
PR, too, is a member of the steroid receptor subgroup of ligand-activated transcription factors within the large nuclear receptor superfamily. It contains 946 amino acids, a DNA-binding domain sandwiched between an N-terminal domain with transcription activation and inhibitory functions and a C-terminal ligand-binding domain. The central DNA-binding domain of PR shows considerable sequence homology to that of ER. The two isoforms, namely PR-A and PR-B, are encoded by the same gene (unlike the isoforms of ER) and are identical except that the truncated PR-A is short of 164 amino acids that are seen at the N-terminal end of PR-B. In the normal breast PR-A and PR-B are coexpressed at equal levels in luminal epithelial cells, suggesting that both proteins are required to mediate physiologically relevant progesterone signaling (i.e., formation of lobular-alveolar structures, modulation of milk synthesis and duct development). In breast cancer, however, predominance of one isoform is common, suggesting that the resultant unbalanced expression of PR-A and PR-B may induce aberrant targeting of genes. PRs are under the control of E2 or related estrogens, and in breast cancer, PR is synthesized by tumor cells that are stimulated by estrogens through an interaction with ER. Thus PR is a surrogate marker of ER activity, and it is rare that PR-positive cells do not also express ER.
Ligand-binding assays (sucrose gradient ultracentrifugation and dextran-coated charcoal assays) were the gold standard for early characterization and measurement of PR. With the advent of monoclonal antibodies to PR, IHC largely replaced the biochemical assays for PR measurement in the mid-1990s.
Several studies demonstrated that IHC was superior to ligand-binding assays and enzyme immunoassays for assessing ER and PR status in primary breast cancer and had equivalent or better ability to predict response to adjuvant endocrine therapy. However, reproducible and reliable IHC assays are essential with proper standardization for meaningful clinical application.
Receptor Status Assessment: Why Is It Important?
The assessment of hormone receptors in primary invasive breast cancer is now mandatory because of its significant clinical therapeutic implications. Both ER and PR are strong predictive factors and relatively weak prognostic factors for response to adjuvant and therapeutic hormonal therapy. The standard of treatment for premenopausal women who have ER-positive breast cancer has been 5 years of tamoxifen. For postmenopausal patients, a minimum of 5 years of adjuvant therapy with an aromatase inhibitor or tamoxifen followed by an aromatase inhibitor (in sequence) had been recommended. Findings from several recent phase III randomized control trials have prompted a shift in these practice guidelines. It is now viewed that 10 years of tamoxifen treatment instead of stopping at 5 years can further reduce recurrence and approximately halve breast cancer mortality during the second decade after diagnosis. It was noted that the therapeutic benefit was directly proportional to the level of ER, with patients with higher expression of ER in the breast cancers demonstrating the most benefit. Also, low ER or PR is associated with a high risk of recurrence after hormonal therapy. On the other hand, ER-negative cancers respond favorably to chemotherapy and are more likely to achieve a complete pathologic response after neoadjuvant chemotherapy than do ER-positive tumors. Interestingly, single hormone receptor-positive tumors (ER-positive/PR-negative and ER-negative/PR-positive) are known to have less sensitivity to tamoxifen, although some reports claim that patients with ER-negative/PR-positive tumors may derive benefit from tamoxifen. Such knowledge underscores the importance of properly identifying patients who truly express ER and PR in their tumors, so that they do not lose the opportunity for timely and appropriate treatment.
It was expected that IHC, like ligand-binding assays, was an intrinsically quantitative method that demonstrated a direct linear relationship between the amount of ER protein present in tumor cell nuclei and the amount of ER antigen detected by IHC. However, a number of studies have shown that this is not the case and that ER IHC results are often highly influenced by preanalytic and analytic factors such as tissue fixation time, antigen retrieval methods, and antigen detection methods. Hormone receptor IHC has been the topic of numerous controversies due to lack of standardization of the test, poor reproducibility among laboratories, and lack of proficiency testing. Standardization of IHC testing and cutoff values for positive results are hence critical to avoid false-negative results. In 2001, an NIH consensus development panel recommended that patients with any expression of hormone receptor in their tumor cells may benefit from hormonal therapy, implying that a mere positive or negative ER status suffices in therapeutic decision-making. Later studies however, emphasized that quantification of hormonal receptors by IHC may help better identify patients who may benefit from adjuvant chemotherapy and also clarify why some patients do not respond to hormonal therapy.
Scoring of Receptor Expression
There has been no uniform method of interpreting IHC results. Although some pathologists use a binary system (completely negative or unequivocally positive), others use a continuous reporting system for ER and PR. Also, no uniformly accepted cutoff point for positivity has been determined. Some laboratories use such arbitrary thresholds as more than 5%, more than 10%, and even 20% for ER-positive tumors. To address these issues, quantification systems have been generated that may use only the proportion of positive cell nuclei or may include the intensity of immunoreactivity as well. The proportion of positive staining cells is a visual estimation, usually depicted as a percentage, whereas staining intensity of cells are reported as weak, moderate, or strong based on the degree of staining characteristics. There is heterogeneity of immunoreactivity in most cancers and the intensity of stained cells are often affected by the actual amount of protein present, the concentration and quality of antibodies used (high or low affinity), and other technical aspects, such as antigen retrieval and detection systems.
In 1985, McCarty and colleagues described the H-score semiquantitative scoring system. The H-score consists of the sum of the percent of tumor cells staining multiplied by an ordinal value corresponding to the intensity level (0 = none; 1 = weak; 2 = moderate; and 3 = strong) with a maximum possible score of 300. According to the modified H-score, a score less than 1 is considered negative, a score less than 100 is weakly positive (1+), a score of 101 to 200 is moderately positive (2+), and a score of 201 to 300 is strongly positive (3+).
The Allred score, described by Allred and colleagues, is calculated by adding a proportion score to an intensity score. The proportion of positive staining cells is scored on a 0 to 5 scale (0 = no staining; 1 = less than 1%; 2 = 1%–10%; 3 = 11%–33%; 4 = 34%–66% and 5 = 67%–100%). The staining intensity of tumor cells is scored on a 0 to 3 scale (0 = none; 1 = weak; 2 = moderate; 3 = strong). These two scores are then added together for a final score of 0 or 2 through 8. A final score of 0 to 2 is considered negative and a score between 3 and 8 are positive. Studies have shown that cancers with an Allred score of 2 had similar outcome compared with patients whose cancers were completely negative for ER. Most breast cancers fall between Allred scores 7 and 8 that show excellent response to treatment. Tumors with scores 3 and 4, although considered positive, are not well studied.
Both the H-score and Allred score are widely used, and they classify tumors to fairly comparable but not identical groups. Among other scoring systems, a modified J-score, introduced by Japanese investigators, only evaluated the number of positive cells without taking the staining intensity into consideration. The criteria used for J-score was as follows, using both 1% and 10% as their cutoff points; J-score 0: no staining; J-score 1: less than 1% stained cells; J-score 2: stained cells more than 1% but less than 10%, and J-score 3: more than 10% stained cells. The final decision on hormone receptor status was classified as negative (J-score 0), Equivocal (J-score 1 and 2), and Positive (J-score 3). This scoring system did not appear to gain acceptance in the Western world, however.
Larger laboratories use computer-assisted image analysis to aid in quantitation of staining in IHC. This is expected to improve interlaboratory variability. Turbin and associates showed that fully automated quantitation of ER immunostaining yielded results that did not differ from human manual scoring against both biochemical assay and patient outcome gold standards. Their cutoff scores were 0: less than 1% positive tumor nuclei, 1+: 1% to 25% positive nuclei, 2+: 25% to 75% positive nuclei, and 3+: more than 75% positive nuclei). The optimal cutoff point found in their study for automated scoring was 0.4% of positive tumor nuclei, consistent with the findings of Harvey and colleagues.
American Society of Clinical Oncology/College of American Pathologists Recommendations
To improve the accuracy of immunohistochemical ER and PR testing in breast cancer and the utility of these receptors as predictive markers, the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) guideline recommendations were announced in 2010. The expert panel determined that when 1% or more of the tumor cell nuclei are immunoreactive to ER (or PR), the test is considered positive, provided expected reactivity of internal and external controls are met. It has been shown that higher ER levels have a higher probability of response to therapy. Although debatable, ER expression as low as 1% positive staining has been associated with clinical response. Therefore reporting low or weak ER expressions in the range of 1% to 10% will allow the clinician to assess the benefits of hormonal therapy versus risks on a case-by-case basis. This was the basis for a ≥1% cutoff choice. According to ASCO/CAP recommendations, less than 1% immunoreactive tumor cells (in the presence of positive internal controls) are considered receptor negative. Allred scores, H-scores, or simply reporting the percentage of positive cells are used for receptor quantitation.
The CAP imposes stringent quality assurance and reporting requirements for ER and PR testing. These include proper validation of tests to ensure accuracy, carrying out laboratory inspections and accreditations, and conducting external proficiency testing surveys. Minimizing cold ischemic time of breast specimens to less than 1 hour and adhering to adequate fixation times (more than 6 hours and less than 72 hours) are also crucial. Patient test reports should list antibody and dilutions used, antigen retrieval methods (if performed), and document the use and appropriate staining of controls.
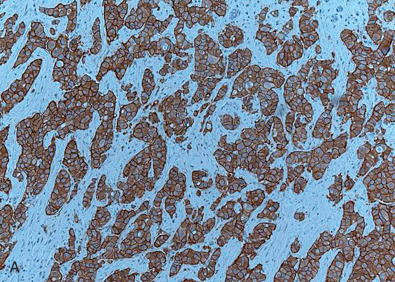
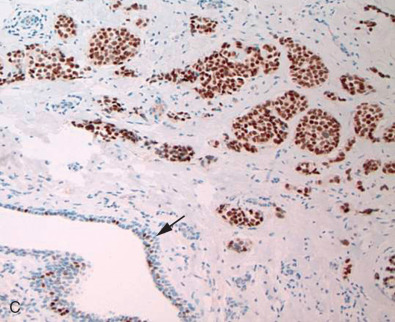
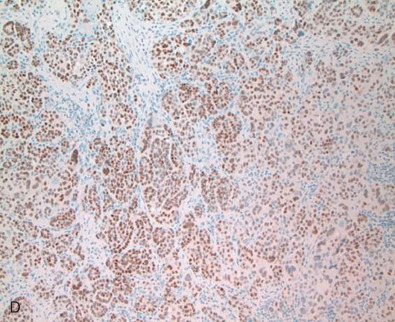
Fig. 14.1 depicts images of four separate invasive breast cancers. Two of them show strong and diffuse ER positivity, the third shows a mixed pattern of ER expression, and the fourth case shows complete absence of ER. Note the presence of ER, marked as nuclear positivity within the benign ductal structure, internal control, confirming antigen preservation.
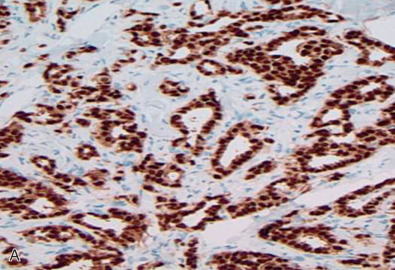
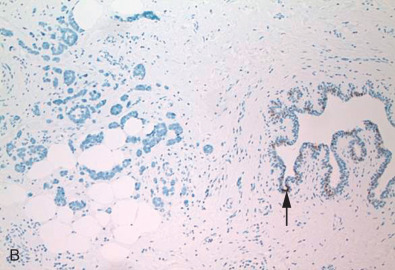
Correlation With Oncotype Dx
With the new concepts of personalized medicine, the past decade has seen the emergence of several gene classifier tests to assess the risk of disease recurrence and thereby tailor individual treatment in breast cancer patients. Oncotype Dx (GenomicHealth, Redwood City, CA) is one such molecular assay with its 21-gene signature (16 prognostic genes that included ER and PR and 5 reference genes) that has gained worldwide acceptance among oncologists. It is a quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) test performed on formalin-fixed paraffin-embedded tissue that generates a recurrence score (ranging from 0 to 100) classifying patients as having low (score <18), intermediate (18–30), or high (>31) risk of disease recurrence. Studies demonstrated that patients with assigned low recurrence score by Oncotype Dx could be spared adjuvant chemotherapy and be treated with tamoxifen alone, whereas patients with higher recurrence scores would benefit from combined tamoxifen and chemotherapy to significantly reduce disease relapse. Ongoing trials attempt to determine the benefits of combined therapy versus hormonal therapy for the Oncotype Dx intermediate recurrence score patients. The test was originally intended for early stage (stages I, II, and IIIa) ER-positive, node-negative invasive breast cancer. With multiple clinical validation studies, this test is now extended for the use of all newly diagnosed early stage, node-negative, or node-positive (1–3 nodes), ER-positive, and HER2-negative breast cancers, although the test’s exact role in the node-positive patients remain controversial.
Oncotype Dx assay with its recurrence score is undoubtedly a valuable prognostic tool in therapeutic decision-making. In addition to the recurrence scores, this test also reports quantitative ER and PR RNA expression levels at internally validated positive cutoff levels (≥6.5 for ER and ≥5.5 for PR). Several studies identified discordance between hormone receptor status detected with IHC and the RT-PCR assay. In both studies there was relatively good (~90%) concordance between IHC and RT-PCR. However, Badve and associates noted in their analysis that IHC ER-negative cases that were RT-PCR positive were more common than IHC ER-positive cases that were RT-PCR negative. In regard to PR, Badve and colleagues and Kraus and associates reported that IHC PR-negative cases that were RT-PCR positive were less common than IHC PR-positive cases that were RT-PCR negative. Kraus and colleagues concluded that IHC was superior to and more sensitive than RT-PCR in detecting low-intensity or heterogeneous expression of hormonal positivity in tumors. They also noted that the technical aspects involved in RT-PCR (RNA extraction techniques, primers, and reagents used as well as tissue microdissection and grinding that could cause potential contamination with normal breast tissue, inflammatory cells, and in situ carcinoma) could result in the discrepancies. In addition, it should be kept in mind that although IHC detects ER and PR at the protein level, RT-PCR detects RNA expression that may or may not always be translated to proteins. Therefore IHC should remain the gold standard in ER and PR analysis in the management of breast cancer patients.
Repeat Immunohistochemical Studies on Recurrent and Metastatic Disease
Hormone receptor testing in all primary breast cancers is now mandatory. However, repeat IHC testing may also be indicated in the settings of recurrent and metastatic breast cancer because alterations of the ER status of tumors have been known to occur over time that could affect treatment decisions. Reports show that the ER status of breast cancer could revert from ER-positive to ER-negative and vice versa in as much as a third of cases. Receptor status conversion generally occurs over long periods of time but has rarely been reported under 1 year. Patients with an ER-negative to ER-positive tumor conversion may benefit from hormonal therapy. However, losing ER status in a recurrent or metastatic disease may signify aggressive disease often resistant to treatment. The ER status of the recurrent or metastatic disease is considered the current ER status of a patient for treatment purposes.
For completeness, other instances that warrant repeat testing for ER and PR are mentioned here. It is strongly advocated to repeat IHC on resection specimens when a core biopsy (with or without appropriate internal positive controls) shows negative results. This is mainly because tumor cells express hormonal receptors in a wide dynamic range with resultant staining heterogeneity that could be missed in small biopsy specimens. Likewise, IHC should be repeated when the background normal breast tissue in a biopsy (that serves as internal positive control) is negative.
ER-negative and PR-positive tumors are rare and sometimes a controversial entity. Therefore it is prudent to repeat ER and PR studies before reporting in such cases.
Commonly Used Monoclonal Antibodies
The original monoclonal antibodies for ER and PR IHC were raised in mouse sera. The different clones of the established mouse antibodies currently available in the market have shown comparable results. Rabbit monoclonal antibodies for ER and PR were introduced in the mid-2000s with the claims that they had higher affinity than their mouse monoclonal counterparts, allowing the use of the antibodies at higher dilutions and in some instances eliminating the need for heat-based antigen retrieval. Cheang and colleagues showed that rabbit monoclonal ER antibody clone SP1 was a better independent prognostic factor than one of the most routinely used mouse monoclonal antibody clone, 1D5. Later studies concluded that the quality and reliability of IHC results achieved with rabbit monoclonal antibodies to ER and PR in invasive breast cancer were comparable to those achieved with established mouse monoclonal counterparts. Newer clones of antibodies are being introduced, and a recent ER rabbit monoclonal clone EP1 was shown to be a highly sensitive and specific antibody. The EP1 resulted in a stronger staining intensity compared with established clone SP1, allowing improved interpretation of ER IHC results.
Tables 14.1 and 14.2 list some of the commonly used monoclonal antibodies for ER and PR. The optimal dilutions for primary antibodies must be determined by the individual laboratories depending on the procedures, antigen retrieval methods, and detection systems used.
| ER Clone | Manufacturer | Source | Suggested Dilution |
|---|---|---|---|
| 1D5 | DakoCytomation, Carpinteria, CA | Mouse | 1 : 10–1 : 50 |
| SP1 | LabVision, Fremont, CA | Rabbit | 1 : 30–1 : 100 |
| 6F11 | Novocastra, Newcastle upon Tyne, UK | Mouse | 1 : 20–1 : 50 |
| EP1 | DakoCytomation, Carpinteria, CA | Rabbit | Ready to use |
| PR Clone | Manufacturer | Source | Suggested Dilution |
|---|---|---|---|
| PgR636 | DakoCytomation, Carpinteria, CA | Mouse | 1 : 50 |
| SP2 | LabVision, Fremont, CA | Rabbit | 1 : 50 |
| 1294 | DakoCytomation, Carpinteria, CA | Mouse | 1 : 50 |
| 1A6 | DakoCytomation, Carpinteria, CA | Rabbit | Ready to use |
Human Epidermal Growth Factor Receptor 2
Historical Perspective
HER2 is a transmembrane tyrosine kinase receptor belonging to a family of epidermal growth factor receptors (EGFRs), encoded by erbB2/HER2-neu oncogene and located on chromosome 17q21. ErbB2 was first discovered as an avian retrovirus oncogene that could induce erythroid leukemia. Researchers found that exposure of neu oncogene transformed NIH 3T3 cells to monoclonal antibodies reactive with the neu gene product p185 resulted in the rapid and reversible loss of both cell surface and total cellular p185. They suggested that p185 was required to maintain the transformation induced by neu oncogene. The erbB/HER2 gene was first sequenced in 1984, and it later was shown that 50% of the amino acids of HER2/neu and the EGFR were identical with greater than 80% homology in the amino acids of their tyrosine kinase domains. Studies also revealed that, although related, HER2 was distinct from EGFR. Amplification of erbB/HER2 gene had been recognized in three separate adenocarcinoma cell lines originating from salivary gland, mammary gland, and gastric carcinoma. These observations indicated that this gene might play an important role in cancer progression.
In 1987 Slamon and his colleagues found that HER2/neu gene was amplified 2- to 20-fold in approximately 30% of the primary human breast cancers they studied. They and others identified that this gene amplification was a significant predictor of both overall survival (OS) and time to relapse in patients with breast cancer. Moreover, multivariate analysis showed HER2/neu amplification had greater prognostic value than other prognosticators like hormonal receptor status with the exception of positive lymph node metastases. Subsequent studies recognized that some of the methods used to evaluate HER2/neu amplification such as solid matrix blotting techniques (Southern, Western, and Northern) could potentially underestimate the number of cancers with amplified genes due to sample contamination caused by adjacent noncancerous tissue. Immunochemistry can be easily employed to assess HER2/neu expression on formalin-fixed paraffin-embedded tissue sections that appear to correlate well with gene expression.
Fendly and colleagues reported data on treating breast cancer cell lines with monoclonal antibodies directed against the extracellular domain of HER2/neu. They demonstrated that the antibodies inhibited the growth of tumor cells and prevented colony formation. Furthermore they showed that the resistance to cytotoxic effects of tumor necrosis factor was significantly reduced in the cells lines treated with monoclonal HER2/neu antibodies. Transfected NIH 3T3 cell lines expressing HER2/neu gene were used to produce and characterize 10 monoclonal antibodies that could immunoprecipitate p185HER2. These monoclonal antibodies would bind to the extracellular domain of p185HER2 and not cross-react with EGFR.
In 1992, researchers humanized antibodies against the 185 kD glycoprotein of HER2/neu that were previously developed in rodents. However, these antibodies were not sufficiently specific to be used in the treatment of breast cancer. In 1994 Pietras and associates showed that anti- p185 HER2/neu in combination with cytotoxic drugs such as cisplatin promoted drug-induced killing of HER2/neu overexpressed tumor cells. This rationale was used to formulate clinical trials.
Genentech (San Francisco, CA), a private biotechnology company, started the phase I clinical trial with recombinant, humanized monoclonal anti-p185 HER2/neu. This antibody was eventually named trastuzumab (Herceptin). After successful enrollment and completion of the phase I clinical trial, the phase II trial was launched in 54 centers in North America, Australia, and New Zealand. Due to amazing success observed in some patients, a phase III trial was initiated before the completion of phase II. However, techniques for identifying HER2/neu-amplified cases were still not standardized. Some laboratories promoted IHC to be performed on frozen tumor and the digitalized images of the immunostained slides analyzed by computer-assisted programs. This methodology was cumbersome and not easily adaptable by all pathology laboratories. By the time the phase III clinical trial was completed, Genentech received US Food and Drug Administration (FDA) approval for marketing trastuzumab. Dako (Carpinteria, CA) received FDA clearance for HercepTest, a semiquantitative immunohistochemical assay for determination of HER2/neu protein overexpression in breast cancer tissues routinely processed for histologic evaluation.
In 1997 Press and colleagues published the validation study of fluorescence in situ hybridization (FISH) for HER2/neu gene from formalin-fixed archival blocks. They showed this technique was superior to Southern blot hybridization with sensitivity of 98% and specificity of 100%. They also demonstrated that HER2/neu gene amplification was an independent factor highlighting poor prognosis in patients who had not received any adjuvant treatment. Several studies confirmed that accurate assessment of HER2/neu was essential to ensure the effectiveness of Herceptin. Multiple studies of metastatic and recurrent breast cancers confirmed that patients with immunohistochemical 3+ or FISH-positive breast cancers gained the greatest clinical benefits. It was particularly impressive that the response rate was superior if trastuzumab was given together with cytotoxic drugs.
At the same time, several researchers recognized that the results of HER2/neu by IHC were not reliable without proper standardization. Treatment of breast cancer was rapidly changing, necessitating standardized surgical pathology reports, including breast biomarker results. At a national consensus conference, breast experts emphasized that the most appropriate methods to identify HER2/neu amplification were IHC and FISH. They also highlighted that PCR-based assays had shown significant association but not complete concordance of HER2/neu results with the preceding methods. They felt there was compelling evidence for routine testing for HER2/neu on all invasive carcinoma. They also reiterated the current methodology for testing had not been optimized, and it was not clear whether FISH was superior to IHC; therefore they recommended, if in doubt, to perform both tests and a conservative approach in interpreting the results. Meanwhile other studies showed that the length of formalin fixation could cause loss of nuclear signals and erroneous HER2 FISH result. Cell lines grown in culture with specific copy of HER2/neu signals and fixed in formalin were introduced to represent an optimal control for HER2/neu tests. A group conducted a retrospective study comparing the result of two separate available immunoassays using the same antibody at two separate institutions. Only 84% concordance could be achieved by IHC. They felt if the tumor showed strong and diffuse cytoplasmic positivity, it was more likely to show HER2/neu gene amplification; however, if the cytoplasmic membrane staining was not strong and diffuse, only 27% showed HER2 gene amplification. They recommended that all breast cancers showing moderate complete cytoplasmic membrane positivity to be tested by FISH. This method was gaining popularity compared with IHC analysis and seemed to predict more accurately clinical responses to trastuzumab-based therapies.
In May 2003 the CAP created a new comprehensive education model, called “Strategic Science,” with expert speakers integrating new and evolving basic, clinical, and scientific issues of HER2/neu testing. In 2006 the CAP published the result of interlaboratory comparison of HER2/neu testing from 2004 to 2005. The comparison studies among the laboratories had been excellent with 90% and 91% consensus. Because the majority of general pathologists do not work fluorescence microscopy, investigators tried to identify new methodologies to recognize gene amplification. One such method was introduced by a group as dual chromogenic in situ hybridization (CISH). They described their method to be superior to single CISH. They suggested their method was more user-friendly for practicing pathologists, and the result showed an almost 100% correlation with FISH results. In 2007 the ASCO and CAP published joint recommendations stating that all invasive breast cancers required HER2/neu testing. The recommended algorithm was introduced for more accurate and reproducible results. They also established a clear algorithm defining positive, equivocal, and negative values for both HER2/neu protein expression and gene amplification.
Standard Practice and New Challenges
Since the FDA approval of HercepTest in 1998 for identifying HER2/neu amplified breast cancers and trastuzumab for treatment of these patients, the scientific community has made tremendous effort to standardize the HER2 testing in breast tissue. Several studies have been performed, reevaluating the HER2 result by examining microarray slides. In the discrepant cases, the entire slide was reexamined. The result has been promising. The actual false-positive rate was 1.3%, and the false-negative rate 0.7%. This result is encouraging for both patients and oncologists. According to the latest recommendation of ASCO/CAP, all newly diagnosed, recurrent, and metastatic breast cancers should be tested for HER2/neu amplification by IHC or CISH. This recommendation was necessary as more laboratories were evaluating gene amplification by bright field, and the original recommendations did not address scoring methods for bright field, mono-color and dual-color in situ hybridization. Additional strategies for detecting HER2/neu amplification were published, including DNA expression by microarray and messenger RNA (mRNA) expression RT-PCR. Because the majority of pathology (CAP or Clinical Laboratory Improvement Amendment [CLIA]-certified) laboratories are following the strict updated guidelines by ASCO/CAP, these issues need to be resolved. The reviewers at the 2013 meeting acknowledged that the treatment of HER2-positive breast cancers by trastuzumab as a single drug or in combination with other cytotoxic drugs has become standard practice. These studies have shown that using trastuzumab would significantly improve OS. The committee members reported that at this point there is insufficient evidence to support use of mRNA or DNA microarray assays to determine HER2 status in unselected patients. The committee members also acknowledged that prior guidelines had not taken into consideration some of the new challenges in evaluating HER2, such as unusual HER2 genotypic abnormalities, aneusomy (monosomy or polysomy) of chromosome 17, colocalization of HER2 and chromosome enumeration probe 17 (CEP17) signals that affect HER2/CEP ratio in dual signal in situ hybridization (ISH) and genetic heterogeneity. Aneusomy 17 (monosomy 17 or polysomy 17) can be detected in approximately 30% of breast cancers. Anomalies in chromosome 17 are common in tumors with discrepant ERBB2 expression and in tumors with discordant ERBB2-protein and ERBB2 gene copy number measurements.
Polysomy defined by the presence of extra copies of one or more whole chromosomes provides an alternative mechanism for apparent HER2 gene amplification. Polysomy of chromosome 17 has frequently been reported in breast cancer. In fact, polysomy has been recognized as a major cause of discrepancy between IHC and ISH results. A study published in 2012 confirmed that the absolute number of HER2/neu signals was important in response to the treatment rather than the ratio of HER2/CEP17. Because the ASCO/CAP committee members intended to reduce or possibly eliminate false-positive and false-negative cases, they felt that a change in reporting HER2/neu positivity was necessary. In the United States, this guideline recommends the use of an FDA-approved assay. CLIA-certified laboratories may choose a laboratory developed test, but they are expected to validate them. A list of FDA-approved assays is available at their website.
Table 14.3 reflects the reporting recommendation for IHC. Table 14.4 reflects the reporting recommendation for FISH/dual bright-field in situ hybridization.
| Score | Pattern |
|---|---|
| Negative (0) | No staining observed or Faint/barely perceptible membrane staining in ≤10% of invasive tumor cells |
| Negative (1+) | Incomplete membrane staining that is faint/barely perceptible within >10% of invasive tumor cells |
| Equivocal (2+) | Complete circumferential membrane staining in ≤10% of invasive tumor cells Incomplete weak to moderate circumferential membrane staining in >10% of invasive tumor cells |
| Positive (3+) | Complete intense circumferential membrane staining in >10% of invasive tumor cells |
| HER2/CEP17 | Average HER2 | Final Result |
|---|---|---|
| Ratio <2 | Average HER2 copy/cell ≥6 | ISH positive |
| Average HER2 copy/cell ≥4 <6 | ISH equivocal | |
| Average HER2 copy/<4 | ISH negative | |
| Ratio ≥2 | Average HER 2 copy/cell ≥4 | ISH positive |
| Average HER 2 copy/cell <4 | ISH positive |
To ensure the quality, all specimens used for HER2/neu testing (cytologic sample, biopsy, or resection) must be fixed within 1 hour, in 10% neutral buffered formalin for duration of 6 to 72 hours. In addition, the laboratories must conform to standards set for CAP accreditation or an equivalent accreditation authority, including initial test validation, ongoing internal quality assurance, ongoing external proficiency testing, and routine periodic performance monitoring. The committee urged pathologists and the laboratories to be more vigilant in eliminating false results because additional HER2-targeted drugs, such as lapatinib and pertuzumab, are shown to have significantly more side effects. These drugs are also more expensive and associated with other dose-limiting side effects. These drugs have shown no clinical benefit in patients with HER2/neu negative metastatic disease.
Several issues remain unresolved. It is still unclear how patients with HER2/neu heterogeneity respond to the anti-HER2 treatment. Many breast cancers show intratumoral heterogeneity. This becomes a challenge for HER2 testing. Two types of HER2 heterogeneity have been described. One shows cluster or colonies of amplified cells. The second group of cancers shows dispersed HER2/neu-amplified cells. By definition, if more than 5% and less than 50% of the cells should show evidence of HER2/neu amplification, the tumor can be classified as showing HER2/neu heterogeneity. The ratio of HER2/Cep17 should be two or more or the neoplastic cells should show more than six HER2/neu signals. Some studies have shown that tumors with less than 80% HER2 positivity of (3+) by IHC or a HER2/Cep17 gene amplification greater than 2.2 recur faster and have lower OS. Evaluation of data published after the 2013 ASCO/CAP recommendation has shown that the number of equivocal and positive cases examined by IHC and CIS had increased. The question remains of how many of these patients will be enrolled in a clinical trial.
Fig. 14.2 shows a composite image of HER2 expression by IHC scored according to the current ASCO/CAP standards.