Bone tumors
Timothy A. Damron, MD, FACS
Overview
Bone tumors are uncommon lesions that affect all ages of patients and may involve any bone in the body. Bone lesions may be benign neoplasms or reactive lesions, primary bone sarcomas, metastatic carcinomas to bone, myeloma, or lymphoma. The benign bone lesions, which predominate in children and young adults, may behave in an inactive, active, or aggressive fashion, and the latter may simulate malignancy. Primary bone sarcomas have a bimodal distribution, whereas metastatic disease, myeloma, and lymphoma of bone predominate in adults. Despite the broad spectrum of bone tumors, each individual entity has a distinct clinical and radiographic presentation, with a predilection for specific locations, which lends itself to narrowing the differential diagnosis and selecting appropriate management.
Bone sarcomas account for less than 0.2% of all cancers. During 2014, approximately 3020 new cases of primary bone sarcomas were diagnosed, and approximately 1460 deaths occurred. The three most common bone sarcomas are osteosarcoma, chondrosarcoma, and Ewing sarcoma. However, the most common primary malignancy of bone is myeloma, and the most common cancer that involves bone is metastatic carcinoma.
The pathologic classification of bone tumors continues to evolve. To a large extent, classification continues to be according to the cell of origin or tissue type. Primary bone tumors may derive from cartilage cells, bone cells, and vascular cells, among others, but for some tumors, the cell of origin is unknown. The most widely accepted pathologic classification system to date is that of the World Health Organization.
Introduction
As a group, bone tumors are uncommon lesions arising from a wide array of cells, affecting all ages of patients, and involving any bone in the body. They include benign lesions, primary bone sarcomas, metastatic carcinomas to bone, myeloma, and lymphoma. The benign bone lesions may behave in an inactive, active, or aggressive fashion. Despite the broad spectrum of bone tumors, each individual entity has a distinct clinical and radiographic presentation, with a predilection for specific locations, which lends itself to narrowing the differential diagnosis and selecting appropriate management.
Bone sarcomas account for less than 0.2% of all cancers.1 During 2014, approximately 3020 new cases of primary bone sarcomas were diagnosed, and approximately 1460 deaths occurred. The three most common bone sarcomas are osteosarcoma (45%), chondrosarcoma (36%), and Ewing sarcoma (18%).2 However, the most common primary malignancy of bone is myeloma, and the most common cancer that involves bone is metastatic carcinoma. Malignancies of bone as a group are only the tip of the iceberg, as the vast majority of bone lesions are benign.
The pathologic classification of bone tumors continues to evolve. To a large extent, classification continues to be according to the cell of origin or tissue type. Primary bone tumors may derive from cartilage cells such as chondrocytes (enchondromas, periosteal chondromas, chondroblastomas, chondromyxoid fibromas, chondrosarcomas), bone cells such as osteoblasts and osteocytes (osteoma, osteoid osteoma, osteoblastoma, osteosarcoma), and vascular cells (hemangioma and angiosarcoma), among others, but for some tumors, the cell of origin is unknown. The most widely accepted pathologic classification system to date is that of the World Health Organization (WHO), and their terminology is used to a large degree in this chapter, although some tumors, such as benign fibrous tumors, are grouped differently.3 The WHO classification of bone tumors has been updated since the last publication of this text (Table 1).
Table 1 Classification of bone tumors by 4th edition of WHO classification on bone and soft-tissue tumors.3
| Chondrogenic tumors |
| Osteochondroma |
| Chondromas: enchondroma, periosteal chondroma |
| Chondromyxoid fibroma |
| Osteochondromyxoma |
| Subungual exostosis and bizarre parosteal osteochondromatous proliferation |
| Synovial chondromatosis |
| Chondroblastoma |
| Chondrosarcoma (grades I–III) including primary and secondary variants and periosteal chondrosarcoma |
| Dedifferentiated chondrosarcoma |
| Mesenchymal chondrosarcoma |
| Clear cell chondrosarcoma |
| Osteogenic tumors |
| Osteoma |
| Osteoid osteoma |
| Osteoblastoma |
| Low-grade central osteosarcoma |
| Conventional osteosarcoma |
| Telangiectatic osteosarcoma |
| Small cell osteosarcoma |
| Parosteal osteosarcoma |
| Periosteal osteosarcoma |
| High-grade surface osteosarcoma |
| Fibrogenic tumors |
| Desmoplastic fibroma of bone |
| Fibrosarcoma of bone |
| Fibrohistiocytic tumors |
| Nonossifying fibroma and benign fibrous histiocytoma of bone |
| Ewing sarcoma |
| Hematopoietic neoplasms |
| Plasma cell myeloma |
| Solitary plasmacytoma of bone |
| Primary non-Hodgkin lymphoma of bone |
| Osteoclastic giant cell-rich tumors |
| Giant cell lesion of the small bones |
| Giant cell tumor of bone |
| Notochordal tumors |
| Benign notochordal cell tumor |
| Chordoma |
| Vascular tumors |
| Hemangioma |
| Epithelioid hemangioma |
| Epithelioid hemangioendothelioma |
| Angiosarcoma |
| Myogenic, lipogenic, and epithelial tumors |
| Leiomyosarcoma |
| Lipoma |
| Liposarcoma |
| Adamantinoma |
| Tumors of undefined neoplastic nature |
| Aneurysmal bone cyst |
| Simple bone cyst |
| Fibrous dysplasia |
| Osteofibrous dysplasia |
| Langerhans cell histiocytosis |
| Erdheim–Chester disease |
| Chondromesenchymal hamartoma |
| Rosai–Dorfman disease |
The introductory sections of this chapter deal with the pretreatment phase (evaluation, staging, and biopsy), the middle sections with surgical treatment (surgical margins through reconstructive options), radiation therapy, and medical management, and the final sections with the specific benign and malignant bone tumors as well as congenital syndromes related to bone tumors.
Evaluation
Crucial information about bone lesions is derived from the history, physical examination, and radiographic features. The goal of evaluation of any bone lesion is to arrive at a narrow differential diagnosis which will guide subsequent action. In some cases, a specific diagnosis may be determined, and, depending upon the diagnosis, the action may include observation (e.g., nonossifying fibroma, enchondroma), biopsy confirmation (e.g., aneurysmal bone cyst (ABC), chondroblastoma, giant cell tumor, osteosarcoma), irrigation/debridement (e.g., osteomyelitis), aspiration/injection (e.g., unicameral bone cyst), radiofrequency ablation (RFA) (e.g., osteoid osteoma), excision (e.g., osteochondroma), or prophylactic stabilization (e.g., established metastatic carcinoma, myeloma). In other cases, the lesion may only be categorized according to a general category of biologic behavior: latent, active, or aggressive. Latent bone tumors may be observed. Active lesions often require biopsy and curettage with or without grafting. Aggressive bone tumors almost always require biopsy confirmation prior to treatment and include both benign aggressive lesions (e.g., ABC, chondroblastoma, and giant cell tumor of bone) and malignancies (e.g., primary bone sarcomas, metastatic carcinoma, myeloma, lymphoma).
Important historical features are the patient’s age and the means by which the lesion was discovered. Age divisions are particularly helpful when the patient is less than 5 years old, where metastatic neuroblastoma has its peak occurrence and sarcomas are rare, and when the patient is older than 40, where the differential diagnosis, in order of decreasing frequency, includes metastatic carcinoma, myeloma, lymphoma, and primary bone sarcomas such as chondrosarcoma, secondary osteosarcoma, malignant fibrous histiocytoma of bone, and fibrosarcoma.
The means of discovery of a bone lesion is variable. Bone lesions discovered incidentally during evaluation for other reasons are usually latent lesions that require nothing further than observation. In adult patients, the most common lesion discovered incidentally is an enchondroma; in pediatric patients, it is a nonossifying fibroma. Painless bony masses are usually osteochondromas, but other surface bone lesions may present in this fashion. The broadest category is the painful bone lesion, and this includes a wide variety of active and aggressive bone lesions. Pathologic fractures may be divided into those preceded by pain, which are usually associated with active or aggressive bone tumors, and those not preceded by pain, which are more commonly latent lesions. Pathologic fractures preceded by pain should usually be biopsied, whereas those not preceded by pain may often be observed at least until the fracture heals, although exceptions exist.
Plain radiographs are standard for the radiologic evaluation of any bone tumor. These should be evaluated for location (epiphyseal, metaphyseal, diaphyseal, surface, or intracortical), lesional border characteristics (type 1 (geographic), type 2 (moth eaten), or type 3 (permeative)), bone response to the lesion (periosteal reaction), and matrix mineralization pattern (cartilaginous, osseous, ground glass) when present (Table 2). Classification of the type 1 border can be broken down into three specific subtypes.5 Type 1A is characterized by a thick, sclerotic margin and usually represents a benign lesion. Type 1B is still well defined but lacks the thick, sclerotic margin, as these lesions potentially have a slightly faster growth pattern and in unusual cases may actually represent low-grade malignancies. Type 1C begins to blend with the moth-eaten pattern, representing a more aggressive pattern, including cortical destruction. Type 1C is characteristic of giant cell tumor of bone or lympoma.6 Hyaline cartilage tumors are one exception to the general rule that benign lesions usually have a sclerotic rim, as most benign enchondromas lack a 1A border. Putting these radiographic features together with the clinical features will often yield a narrow differential diagnosis.
Table 2 Classic examples of bone tumors fitting specific patterns of lesional borders, bone response, and lesional matrix on plain radiographs
| Radiographic category | Type | Classic examplesa |
| Lesional border | Geographic (well defined) | 1A Nonossifying fibroma Unicameral bone cyst |
| 1B Aneurysmal bone cyst Chondroblastoma | ||
| 1C Giant cell tumor Lymphoma | ||
| Moth eaten (blurred) | Metastatic carcinoma Myeloma Lymphoma Osteomyelitis | |
| Permeative (poorly defined) | Osteosarcoma Ewing sarcoma Metastatic carcinoma Lymphoma | |
| Bone response | Marginal sclerosis | Nonossifying fibroma Fibrous dysplasia |
| Cortical thickening | Osteoid osteoma Osteomyelitis | |
| Laminar periosteal response | Stress fracture | |
| Endosteal expansion and scalloping | Low- to intermediate-grade chondrosarcoma | |
| Periosteal rimming | Aneurysmal bone cyst Giant cell tumor of bone | |
| Codman’s triangle Cumulus cloud reaction | Osteosarcoma | |
| Onion-skinning periosteal response | Ewing sarcoma | |
| Lesional matrix | Punctate rings and arcs | Hyaline cartilage tumors (enchondromas/chondrosarcomas) |
| Ground-glass appearance | Fibrous dysplasia | |
| Osteoblastic | Osteoid osteoma Osteoblastoma Osteosarcoma Metastatic carcinoma (prostate, breast) Lymphoma |
a None of the radiographic findings here are specific, and there is considerable overlap; hence, those lesions listed may be considered classic but by no means the only ones that may present with such findings.
Source: Damron 2008.4 Reproduced with permission of Wolters Kluwer Health.
Bone scans help to determine whether the lesion shows uptake of the Tc99 radionuclide, indicating an active or aggressive lesion, and whether the lesion is solitary or only one of numerous lesions. Myeloma deposits in bone serve as an exception to the rule, often being cold on bone scan. Some aggressive lesions, such as renal carcinoma metastases, may not be hot on bone scan if tumor destruction outpaces the bone’s ability to form bone in response.
Positron emission tomography (PET) has come to play an increasing role in evaluation and staging of bone lesions.6 On average, PET avidity is greater for malignant bone tumors than for benign bone tumors. However, 18F-FDG PET avidity has been shown to be nonspecific for malignancy, and many benign tumors, ranging from nonossifying fibroma to osteoid osteomas, also show this finding, albeit with greater variability. PET scans have come to play an increasing role in staging for malignant bone tumors, but they are less sensitive than routine chest computerized tomography (CT) for pulmonary metastases.6
Magnetic resonance imaging (MRI) of bone tumors is indicated when the diagnosis is not evident from the plain radiographs and for determining the local extent of bone sarcomas. Specific bone tumors that may be confirmed with MRI include simple bone cysts (rim enhancement of a fluid-filled lesion) and ABCs (septations separating multiple loculated areas filled with blood, as indicated by fluid-fluid levels). Perilesional edema is common surrounding bone sarcomas but may also be seen in certain benign conditions, including osteomyelitis, osteoid osteoma, chondroblastoma, Langerhans cell histiocytosis (LCH), and chondromyxoid fibroma. CT is indicated to find the radiolucent nidus of an osteoid osteoma within the surrounding reactive bone, to assess for endosteal scalloping in a cartilage tumor to distinguish enchondroma from chondrosarcoma, to find the pattern of lesional mineralization when it is unclear, and to supplement MRI in difficult anatomic locations such as the sacrum, pelvis, and scapula.
Staging
Staging of bone sarcomas requires assessment of both the primary site and distant disease. For assessment of the primary site, radiographs and MRI of the entire involved bone should be obtained in order to check for other “skip” lesions. The two most common sites of metastases from bone sarcomas are lung followed by bone. Hence, the most crucial staging studies to obtain are chest radiograph and CT to evaluate the lungs as well as a total body bone scan or PET scan to evaluate the rest of the skeleton.
Traditionally, the staging system most commonly used for musculoskeletal sarcomas was that originally described by Doctor William Enneking and adopted by the Musculoskeletal Tumor Society (MSTS) (Table 3). Variables incorporated into the MSTS staging system include the presence or absence of metastases, grade (high or low), and local extent of the tumor (intraosseous or with extension into the soft tissues). The typical chondrosarcoma is low grade and intracompartmental (confined to the bone), so it is usually stage IA. The conventional high-grade osteosarcoma usually extends into the soft tissue, and since 80% without evidence of distant metastases, the typical stage is IIB. Evidence of metastases equates to a stage III in this system.
Table 3 Musculoskeletal Tumor Society staging system
| Stage | Grade | Local extent | Metastases |
| I | Low | A – intracompartmental | None |
| B – extracompartmental | |||
| II | High | A – intracompartmental | |
| B – extracompartmental | |||
| III | Any | Any | Present |
Source: Enneking 1980.7 Reproduced with permission from the Association of Bone and Joint Surgeons.
The current most widely accepted staging system for bone sarcomas is that of the American Joint Committee on Cancer (AJCC)8 (Table 4). This system has more well-documented prognostic significance (Figure 1). Variables incorporated into the AJCC system include presence or absence of metastases (with multiple/discontinuous bone tumors separated from distant metastases), grade (grade 1 or 2 vs. grade 3 or 4), and size (8 cm or smaller vs. larger than 8 cm). This system is similar to the MSTS system in separating stage I from II based on grade, but the A–B designation is based upon size here. For osteosarcoma and Ewing sarcoma, stage IIB patients have higher rates of metastases than stage IIA patients. Patients with “skip lesions” (discontinuous lesions in the same bone) are designated as stage III. Distant metastases in this system are stage IV, but since metastases to sites other than the lung (such as bone) carry a worse prognosis than lung metastases patients alone, a separate designation is reserved for those two groups (IVA and IVB). Hence, for a less than 8 cm low-grade chondrosarcoma, the typical staging would be stage IA. For a larger than 8 cm high-grade osteosarcoma without skip lesions or metastases, the typical staging is stage IIB. There were no changes in the AJCC staging between the 2002 and 2012 versions.10
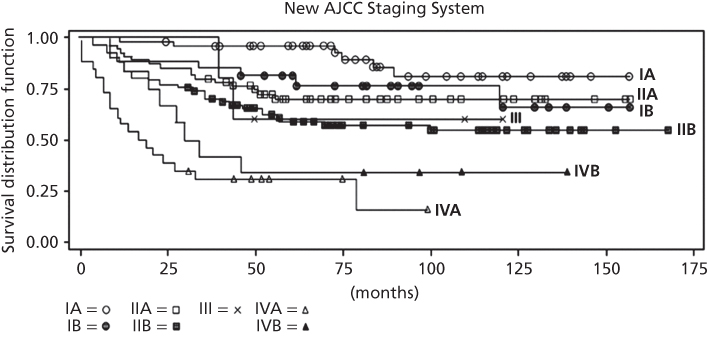
Figure 1 Survival according to staging by the new American Joint Committee on Cancer staging system for bone sarcomas.
Source: ACS 2014.1 Reproduced with permission of The American Cancer Society.
Table 4 American Joint Committee on Cancer staging system for bone sarcomas
| Primary tumor [T] | ||||
| TX | Primary tumor cannot be assessed | |||
| T0 | No evidence of primary tumor | |||
| T1 | Tumor 8 cm or less in greatest dimension | |||
| T2 | Tumor more than 8 cm in greatest dimension | |||
| T3 | Discontinuous tumors in the primary bone site | |||
| Regional lymph nodes [N] | ||||
| NXa | Regional lymph nodes cannot be assessed | |||
| N0 | No regional lymph node metastasis | |||
| N1 | Regional lymph node metastasis | |||
| Distant metastasis [M] | ||||
| MX | Distant metastasis cannot be assessed | |||
| M0 | No distant metastasis | |||
| M1 | Distant metastasis | |||
| M1a | Lung | |||
| M1b | Other distant sites | |||
| Histologic grade [G] | ||||
| GX | Grade cannot be assessed | |||
| G1 | Well differentiated—low grade | |||
| G2 | Moderately differentiated—low grade | |||
| G3 | Poorly differentiated—high grade | |||
| G4 | Undifferentiated—high grade | |||
| Stage | Tumor [T] | Node [N] | Metastasis [M] | Grade [G] |
| Stage IA | T1 | N0 | M0 | G1, 2 low grade |
| Stage IB | T2 | N0 | M0 | G1, 2 low grade |
| Stage IIA | T1 | N0 | M0 | G3, 4 high grade |
| Stage IIB | T2 | N0 | M0 | G3, 4 high grade |
| Stage III | T3 | N0 | M0 | Any G |
| Stage IVA | Any T | N0 | M1a | Any G |
| Stage IVB | Any T | N1 | Any M | Any G |
| Any T | Any N | M1b | Any G | |
a Because of the rarity of lymph node involvement in sarcomas, the designation NX may not be appropriate and could be considered N0 if no clinical involvement is evident. Ewing sarcoma is classified as G4.
Source: Edge 2010.9 Reproduced with permission from Springer.
Biopsy
Biopsy of bone tumors is indicated when a specific benign diagnosis cannot be determined based by radiographic evaluation alone. When the clinicoradiographic diagnosis of a latent lesion such as nonossifying fibroma, unicameral bone cyst, or enchondroma can be made, biopsy is generally unnecessary. Some active lesions, such as fibrous dysplasia, intraosseous lipoma, and osteochondroma do not always require biopsy if the diagnosis can be established radiographically. For most other active and all aggressive lesions, biopsy should be done to determine or confirm the diagnosis. Suspected high-grade sarcomas should be biopsied prior to initiating treatment neoadjuvant.
Careful consideration must be given when metastases are suspected, because the treatment of metastatic carcinoma to bone differs dramatically from that of a primary bone tumor. Hence, even for patients with a history of a known carcinoma with predilection to bone (breast, prostate, lung, renal, thyroid), the first bone metastases should generally be established by biopsy unless the clinical situation represents a terminal state (known wide metastases to other sites). When dealing with suspected metastatic disease requiring a biopsy, reamings are not the best way to submit a biopsy specimen, because once the intramedullary canal to and through the lesion has been breached, all associated bone and soft tissue has been potentially contaminated with tumor. If the pathology shows sarcoma, rather than metastatic disease, unnecessary contamination of previously uninvolved tissue will have already occurred.
There are four general biopsy techniques applicable to bone tumors: (1) fine needle biopsy, (2) core needle biopsy, (3) incisional open biopsy, and (4) excisional biopsy. Fine needle aspiration (FNA) biopsy provides only cells for cytology and is usually done by interventional radiologists. An FNA is useful in two general clinical situations where bone tumors are involved: (1) when the diagnosis of a bone lesion is strongly suspected (metastatic carcinoma, recurrent sarcoma) and (2) for difficult-to-access lesions (spine, pelvis, scapula). Core needle biopsies allow for interpretation of the tissue architecture in addition to the cytological detail. For bone lesions, core biopsies may be done in the same situations as FNA and for bone lesions with soft-tissue extension. Incisional open biopsy is the workhorse biopsy tool for bone lesions, as it provides adequate tissue for histological interpretation. However, there are numerous pitfalls to open biopsy which must be considered, and for most incisional biopsies, the surgeon who will be doing the definitive surgery, no matter what the final diagnosis, is the person who should do the biopsy.11, 12 Excisional biopsy is usually only used for bone tumors such as osteochondromas, where the radiographic features establish the diagnosis, the morbidity of excision is small, and the surface location of the lesion lends itself to excision.
Surgical margins
Surgical margins depend upon the type of excision done, and the appropriate type of excision varies according to bone tumor type. There are four types of excisions: (1) intralesional, (2) marginal, (3) wide, and (4) radical.13 For most benign bone lesions, an intralesional excision by way of a curettage is appropriate. When necessary, metastatic bone lesions undergoing surgery for stabilization may be treated by curettage. For aggressive benign lesions (ABC, chondroblastoma, and giant cell tumor), an extended intralesional curettage is indicated. This differs from a simple curettage in that it utilizes mechanical (high-speed burr, pulsatile lavage) and adjunctive (phenol, laser, liquid nitrogen, argon beam coagulation) techniques to extend the margin into normal bone. Recent trends suggest that most extremity low-grade chondrosarcomas may also be treated in this way. Marginal en bloc excision (through the pseudocapsule surrounding the tumor) is appropriate for osteochondromas, but wide resection (excision with a cuff of normal tissue) is indicated for most bone sarcomas.
Limb salvage versus amputation
Two issues must be considered in making the decision regarding limb salvage versus amputation: oncologic safety and function. First and foremost, in order for a patient to be a candidate for limb salvage, the oncologic procedure should not lower the expected survival beyond that which could be achieved with an amputation. This question has been addressed prospectively, and—in properly selected patients—survival is no less with limb-sparing surgery when compared to amputation.14, 15 However, this decision still needs to be made carefully for each patient. Oncologic safety considers two variables: response to chemotherapy (when applicable) and surgical margin. The poorer the response to chemotherapy, the greater the likelihood of local recurrence for any given margin achieved intraoperatively. Hence, for a poor response to chemotherapy (tumor progression), limb salvage surgery is a relatively strong contraindication. A wide surgical margin is the goal of bone sarcoma surgery, and it can almost always be achieved with the appropriately planned level of amputation but not always with limb-sparing surgery. If vital structures (major vessels and nerves) are encased by the soft-tissue extent of the sarcoma, necessitating their resection along with the tumor, limb salvage is contraindicated and amputation is preferable.
As with oncologic safety, if limb salvage is to be done, the expected function of the planned reconstruction should be at least equivalent to that of a comparable level amputation. In the lower extremity, function with a below-knee amputation is generally thought to be better than a distal tibial reconstruction, so a distal tibial location is a relative indication for amputation. In the upper extremity, it is preferable to be able to preserve 2 of the 3 major nerves (radial, ulnar, median) for limb salvage. Tumor encasement of more than one major upper extremity nerve and/or the axillary or brachial vessels is an indication for amputation.
Operative management of metastatic carcinoma, myeloma, and lymphoma
There are four settings that potentially require operative management for patients with bone lesions from metastatic carcinoma, myeloma, and lymphoma: (1) biopsy to establish diagnosis, (2) prophylactic stabilization of impending pathologic fracture, (3) operative fixation of pathologic fracture, and (4) en bloc resection of isolated lesions. Biopsy issues have been previously discussed but cannot be emphasized enough: the diagnosis should be established before embarking upon an operative treatment plan. Prediction of impending fracture risk is evolving. Currently, fractures are predicted based upon clinical and radiographic criteria. A rating system devised by Mirels has been devised based upon four variables16 (Tables 5 and 6). The Mirels system is valid across experience levels, but it still has a low specificity of approximately 33%.17 Evaluation of CT-based biomechanical analyses for this purpose are under study.18
Table 5 Mirels scoring system for predicting risk of pathologic fracture in metastatic disease
| One point | Two points | Three points | |
| Site | Upper extremity | Lower extremity | Peritrochanteric |
| Size | <1/3 | 1/3–2/3 | >2/3 |
| Nature | Blastic | Mixed | Lytic |
| Pain | Mild | Moderate | Functional |
Source: Mirels 1989.16 Reproduced with permission from Wiley.
Table 6 Mirels definitions, fracture risk, and treatment recommendations based upon point totals
| Definition | Points | Fracture risk (%) | Recommendation |
| Nonimpending | ≤7 | <10 | Observe |
| Borderline | 8 | 15 | Consider fixation |
| Impending fracture | 9 | 33 | Prophylactic fixation |
| Impending fracture | ≥10 | >50 | Prophylactic fixation |
Source: Mirels 1989.16 Reproduced with permission from Wiley.
When pathologic fractures occur in the setting of disseminated malignancies, they usually warrant operative fixation in order to improve function since these patients have limited life expectancy. Exceptions include moribund immediately preterminal patients, those who cannot tolerate operative intervention, and fractures that are usually managed by nonoperative means. In order for the patient to benefit from the procedure, life expectancy should be longer than the expected time required to recover from any proposed procedure.
The principles of operative fixation for fractures in this clinical situation differ from those of standard fracture fixation. Since later lesions may develop elsewhere in the bone, intramedullary nail fixation, as opposed to plate/screw fixation, is preferred in the long bones in order to protect the remainder of the bone. Immediate stability is the goal in order to avoid prolonged recovery that diminishes patient function, so bone cement is much more commonly used to supplement fixation in this situation. Fracture healing in the setting of metastatic carcinoma and myeloma is notoriously slow, so the fixation should be planned assuming there will be no fracture healing. Postoperative radiotherapy has been shown to improve function and reduce reoperation rates.19
Reconstructive alternatives
Benign bone tumors
The defects created after curettage of benign bone tumors can be filled with autologous bone graft, allograft bone, synthetic filler material, or bone cement.20 Bone cement is usually reserved for defects created after extended intralesional curettage of giant cell tumors of bone. Bone cement provides immediate stability that allows full weight bearing, solidifies with an exothermic reaction that extends the margin, and provides a clear radiographic border to facilitate diagnosis of local recurrence. In cases where large lesions have been curetted, prophylactic stabilization with pins or plates/screws may be used to minimize the chance of fracture.
Primary bone sarcomas
Following resection of bone sarcomas, numerous alternatives are available to reconstruct the large bone and associated soft-tissue deficits that result. Selection of the appropriate reconstruction in each instance requires consideration of the patient age and expectations, prognosis, adjuvant treatments, type of resection, and anatomic site. In general, reconstructive techniques include endoprostheses, structural allografts, allograft–prosthetic composites, and vascularized bone grafts. Over time, the use of endoprosthetic and allograft–prosthetic composite reconstructions following resections that include a joint has increased, while the indications for structural allografts have continued to decline. Apart from allograft–prosthetic composite reconstructions, the main role for structural allografts has been for intercalary reconstructions (when the joints above and below the diaphyseal segment can be preserved). The most frequent type of resection for sarcomas is intra-articular (removal of the bone up to and including the joint surface), requiring reconstruction of the joint surface, usually with a joint replacement. When the tumor invades the joint, an extra-articular resection (removal of both sides of the joint) is indicated, and this is often better reconstructed with a joint fusion using intervening allograft bone.
Patient age is a major consideration, since skeletally immature patients will develop limb length discrepancy unless the reconstruction accommodates the loss of growth on the operative side. For patients less than 8 years old, the potential limb length discrepancy is so profound that standard means of reconstruction is generally contraindicated. In these difficult situations, amputation, rotationplasty, and vascularized fibula grafting with open growth plates are viable alternatives.21–27 Rotationplasty in the lower extremity after resection of a tumor around the knee involves fixing the foot and ankle in a position rotated 180 degrees from normal so that the heel points forward and the ankle is situated at the level of and functions as the patient’s knee. In this situation, the patient is able to function as a below-knee amputee rather than having to settle for a higher above-knee amputation. In patients older than 8 years old, expandable endoprosthetic reconstructions are available.28–32 In recent years, the availability of such growing prostheses with a noninvasive mechanism has increased their attraction.31 However, the complication rates have proven to be high, and their durability once the patient reaches skeletal maturity has been less than optimal.33
Upper extremity
For reconstructions following bone sarcoma resection about the shoulder, function is most dependent on whether the deltoid muscle may be preserved during resection of the tumor.34, 35 If a tumor of the proximal humerus extends into the deltoid and the deltoid has to be removed to achieve a wide margin, function of the shoulder will be poor regardless of the reconstruction. When the deltoid can be preserved, consideration is often given to using an allograft–prosthetic composite reconstruction.36 In this way, the patient’s remaining rotator cuff tendons can be repaired to the allograft tendons, and there is potential for improved function. If an osteoarticular allograft reconstruction is chosen, there is an increased risk of nonunion of the allograft–host junction, fracture, and subchondral collapse. The scapula and all portions of the humerus may be reconstructed with an endoprosthesis. The distal upper extremity is an unusual site for bone sarcomas.
Lower extremity
Following limb-preserving bone sarcoma resection of the pelvis (internal hemipelvectomy), no reconstruction is needed when the hip joint is able to be preserved (e.g., anterior pubic/ischial rami and supra-acetabular iliac resections), and some surgeons do not reconstruct even following resection of the acetabular portion of the pelvis. The numerous reconstructive alternatives for the acetabulum and hip joint following internal hemipelvectomy are fraught with complications.37, 38 For the proximal femur, reconstruction is often done with either an endoprosthetic hemiarthroplasty (replacing the femoral head side but without a cup in the pelvis) or an allograft–prosthetic composite.39–41 The latter reconstruction allows attachment of the patient’s hip abductors to the allograft tendon, which may improve stability and gait.
Most reconstructions of the distal femur utilize a distal femoral replacement endoprosthetic total knee reconstruction. Because of the attachment of the extensor mechanism through the patellar tendon to the tibial tuberosity, resection of the proximal tibia necessitates consideration of extensor mechanism reconstruction. Here, the primary alternatives are endoprosthetic proximal tibial total knee reconstruction or allograft–prosthetic composite reconstruction. For both reconstructions, the medial head of the gastrocnemius muscle is often used both to cover the allograft and/or prosthesis and to reconstruct the extensor mechanism. With an allograft–prosthetic composite reconstruction of the proximal tibia, the patient’s remaining patellar tendon may be sutured to the allograft tendon, further augmenting the extensor mechanism reconstruction.
Complications
Complications after resections and reconstructions for bone sarcomas are numerous and frequent. Infection is a concern with all reconstructions, particularly considering the large dead space created following these procedures, the prolonged wound healing while receiving adjuvant treatments, and the prevalence of chemotherapy-induced neutropenia. However, certain complications are associated with specific anatomic sites and types of reconstructions. The proximal tibia is an anatomic site particularly prone to infection and wound breakdown given the paucity of soft-tissue coverage. For endoprosthetic reconstructions of the shoulder and hip, joint instability and frank dislocation are relatively common complications. All endoprosthetic reconstructions are prone to loosening, but prosthetic survival is acceptable (proximal femur (90%), distal femur (60%), proximal tibia (50%)).42 Exciting new developments in alternative compliant means of fixation other than those commonly used with joint arthroplasties have been reported and warrant continued close follow-up.43 Reports of their use around the knee show 80% 10-year survivorship.44 Allografts, particularly when used alone, are prone to nonunion at the allograft–host junction and to fracture.
Radiotherapy for bone tumors
Radiotherapy plays a limited role in the treatment of primary tumors of bone, but it is a mainstay of local treatment for bone lesions resulting from metastatic carcinoma, myeloma, and lymphoma. Radiotherapy should generally be avoided in the treatment of benign bone tumors, but low-dose radiotherapy has been advocated in recalcitrant symptomatic spine cases of LCH.45 For bone sarcomas, radiotherapy may be considered for the treatment of Ewing sarcoma, but it is not part of standard treatment for osteosarcoma or chondrosarcoma.
Potential complications of bone irradiation include postradiation sarcoma, spontaneous and fragility fracture, osteonecrosis, and—in pediatric patients—growth arrest or angular deformities. Postradiation sarcomas typically occur at a minimum of 3 years following the radiation exposure and after mean doses of 50 Gy.46 Risk factors for postradiation fracture include periosteal stripping, neoadjuvant chemotherapy, femoral location, higher-dose irradiation, and circumferential irradiation.47 Postradiation fractures are gaining increasing attention because of their difficulty in management, prolonged healing times and high nonunion rates (45–67%), and the frequent need for surgical treatment, multiple operations, radical bone resection/reconstruction, and/or amputation.47
Medical management of bone tumors
The roles for medical management of bone tumors continue to expand. As a rule, high-grade bone sarcomas warrant chemotherapy to address systemic microscopic disease. Chemotherapy for bone sarcomas is usually initiated prior to (neoadjuvant chemotherapy) and completed after local surgical or radiation treatment. Hence, the treatment of both conventional high-grade osteosarcoma and Ewing sarcoma begins with neoadjuvant chemotherapy. There is no established role for chemotherapy in the treatment of low-grade chondrosarcoma.
Use of bisphosphonates to inhibit osteoclast-mediated bone destruction has become standard of care for myeloma and many metastatic carcinomas, including breast, prostate, and lung.48 Recently, bisphosphonate drugs have also been used off-label for specific benign bone lesions, including fibrous dysplasia and giant cell tumor, but their efficacy has not been established. Of concern, however, is the risk of bisphosphonate-related osteonecrosis of the jaw and atypical subtrochanteric proximal femur fractures.48, 49
Specific benign bone tumors
Cartilage tumors
Cartilage tumors can be divided into those that derive from mature hyaline cartilage (chondromas and osteochondromas) versus those that derive from immature cartilage (chondroblastoma and chondromyxoid fibroma).
Chondromas
The WHO classification lists enchondromas and periosteal chondromas under the general heading of “Chondromas.”3 Enchondromas are the intramedullary variety of these benign hyaline cartilage tumors, and periosteal chondromas are the variety that reside on the bone surface. Enchondromatosis (multiple enchondromas/Ollier’s disease and Maffucci’s syndrome) is discussed under the section titled “Congenital syndromes.” The latter represents multiple enchondromas combined with hemangiomas.
Enchondromas are relatively common among primary bone tumors, representing up to 17% overall. They likely represent residual rests of hyaline growth plate cartilage left behind during skeletal immaturity. Most of these lesions are asymptomatic and incidentally noted on imaging studies done for other causes of pain.50 Enchondromas represent the most common bone lesion seen in the small bones of the hands and feet. In the long bones, they are most commonly located in the femur (where they are frequently picked up on X-rays of patients with hip or knee pain) and the humerus (where they are picked up during radiologic evaluation of patients with common causes of shoulder pain). Solitary enchondromas of the flat bones are rare, and the possibility of chondrosarcoma needs to be considered when a cartilage lesion occurs there. Because the radiographic characteristics of hyaline cartilage are fairly typical, the primary challenge is distinguishing enchondromas from chondrosarcomas.
The natural history of enchondromas is that they become more calcified over time. Hence, mature lesions in adults almost always display a characteristic punctate pattern of calcified arcs and rings which is so distinctive as to often allow the diagnosis to be confirmed radiographically without biopsy (Figure 2). They can be associated with some endosteal scalloping. However, when they have periosteal reaction, expansion of the surrounding bone, more extensive cortical destruction, or soft-tissue extension, they have to be considered chondrosarcomas. MRI characteristics of enchondromas are a lobular pattern of organization which is dark on T1-weighted (T1W) images and bright on T2-weighted (T2W) images. Increased uptake on bone scan is typical of enchondromas and should not be considered to be a sign of malignancy; it is likely due to ongoing remodeling of the surrounding bone.
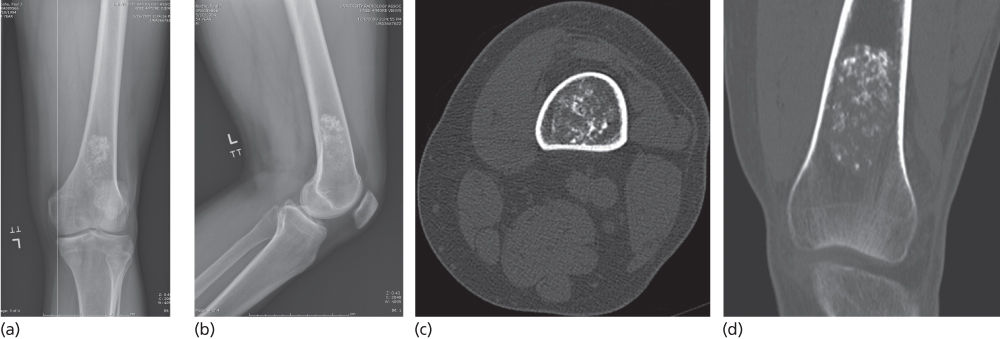
Figure 2 Enchondroma. Anteroposterior (a) and lateral (b) distal femur radiographs as well as axial (c) and coronal (d) CT scans show the characteristic punctate arcs and rings of hyaline cartilage without prominent endosteal scalloping or cortical destruction to suggest features of chondrosarcoma.
Under the microscope, enchondromas are comprised of benign, sparsely cellular hyaline cartilage, but the degree of cellularity and atypia is variable. In certain locations, such as the fingers and other small bones, the histologic features often appear more aggressive despite their benign behavior.
The vast majority of enchondromas, especially when they present as asymptomatic lesions with typical radiological findings, can simply be observed to ensure that they do not progress. However, when either the radiographic features or associated pain cast doubt on the diagnosis, the lesion may require a complete curettage and complete histological review to differentiate from chondrosarcoma. Cartilage lesions rarely recur after curettage.
Periosteal chondromas, by contrast, are distinctly unusual benign hyaline cartilage lesions. They more commonly present as a bump on a digit or as a low-grade painful lesion elsewhere. Radiographically, they usually show a typical “saucerization” of the underlying cortex of this surface lesion. They need to be distinguished from periosteal chondrosarcoma. Smaller lesions (less than 7 cm) are usually chondromas. Depending upon the location, periosteal chondromas may be removed by curettage or en bloc excision.
Chondroblastoma
This immature cartilage-derived tumor is an uncommon benign process that typically presents with joint pain, is difficult to diagnosis, and occurs in the epiphysis of the long bones.51 The vast majority of cases occur in skeletally immature patients, and their presentation with joint pain (due to their location at the end of long bones), mimicking symptoms of far more common processes, and their initially subtle radiographic findings may lead to a prolonged course prior to diagnosis. Although they may occur in numerous bones, their most common locations are the proximal humerus, distal femur, and proximal tibia.
Radiograph appearance can be subtle, since its immature cartilage usually does not cause the calcification seen so characteristically with hyaline cartilage lesions. Furthermore, the subtle epiphyseal round radiolucency of chondroblastoma is not usually surrounded by a very obvious sclerotic rim. Easier to recognize on MRI, chondroblastoma is dark on T1W and bright on T2W images and shows extensive perilesional edema. Bone scans are hot at the site of the lesion.
Chondroblastoma histology shows a preponderance of rounded cells with distinct folded “coffee-bean” nuclei arranged in a pseudolobulated “cobblestone” pattern of organization. Giant cells and chondroid matrix may also be seen.
Treatment of chondroblastoma must take into account the fact that while it usually behaves only as an active lesion, it may also behave in an aggressive fashion. An extended intralesional curettage remains the classic treatment of choice, but more recently RFA has been employed in selected lesions with considerable success.52 When RFA is used for large lesions beneath weight-bearing articular surfaces, the risk of collapse must be considered.52 Chondroblastoma is also one of two benign bone lesions (along with giant cell tumor) that carry the potential to develop pulmonary metastases, so initial screening and subsequent surveillance of the lungs should be considered.
Chondromyxoid fibroma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree