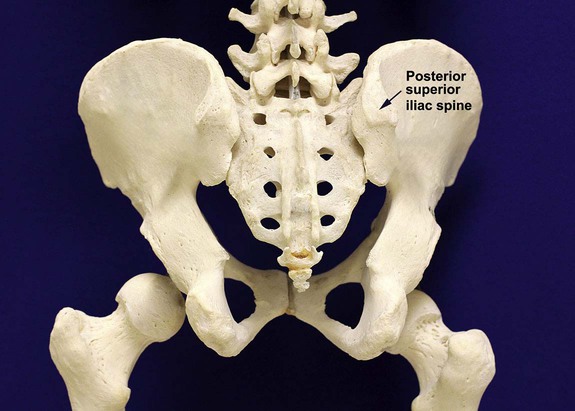
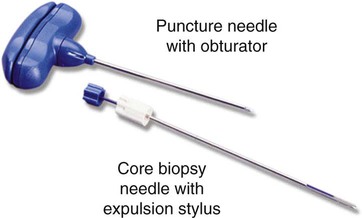
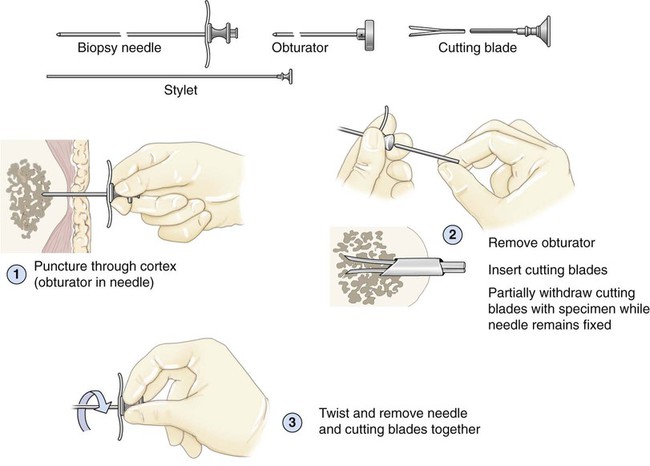
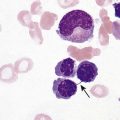
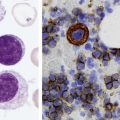
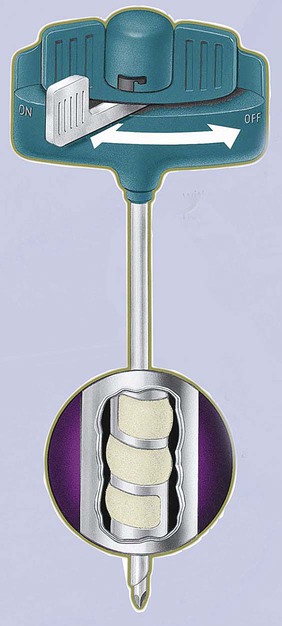After completion of this chapter, the reader will be able to: 1. Diagram bone marrow architecture and locate hematopoietic tissue. 2. List indications for bone marrow examinations. 3. Specify sites for bone marrow aspirate and biopsy. 4. Assemble supplies for performing and assisting in bone marrow specimen collection. 5. Assist the physician with bone marrow sample preparation subsequent to collection. 6. List the information gained from bone marrow aspirates and biopsy specimens. 7. Perform a bone marrow aspirate smear and core biopsy specimen examination. 8. List the normal hematopoietic and stromal cells of the bone marrow and their anticipated distribution. 9. Perform a bone marrow differential count and compute the myeloid-to-erythroid ratio. 10. Characterize features of hematopoietic and metastatic tumor cells. 11. Prepare specimens for and assist in performing specialized confirmatory bone marrow studies. 12. Prepare a systematic written bone marrow examination report. In adults, bone marrow accounts for 3.4% to 5.9% of body weight, contributes 1600 to 3700 g or a volume of 30 to 50 mL/kg, and produces roughly six billion blood cells per kilogram per day in a process called hematopoiesis.1 At birth, nearly all the bones contain red hematopoietic marrow (see Chapter 7). In the fifth to seventh year, adipocytes (fat cells) begin to replace red marrow in the long bones of the hands, feet, legs, and arms, producing yellow marrow, and by late adolescence hematopoietic marrow is limited to the lower skull, vertebrae, shoulder, pelvic girdle, ribs, and sternum (see Figure 7-2). Although the percentage of bony space devoted to hematopoiesis is considerably reduced, the overall volume remains constant as the individual matures.2 Yellow marrow reverts to hematopoiesis, increasing red marrow volume, in conditions such as chronic blood loss or hemolytic anemia that raise demand. The arrangement of red marrow and its relationship to the central venous sinus is illustrated in Figure 7-3. Hematopoietic tissue is enmeshed in spongy trabeculae (bony tissue) surrounding a network of sinuses that originate at the endosteum (vascular layer just within the bone) and terminate in collecting venules.3 Adipocytes occupy approximately 50% of red hematopoietic marrow space in a 30- to 70-year-old adult, and fatty metamorphosis increases approximately 10% per decade after 70.4 Table 16-1 summarizes indications for examination of bone marrow.5 Bone marrow examinations may be used to diagnose and stage hematologic and nonhematologic neoplasia, to determine the cause of cytopenias, and to confirm or exclude metabolic and infectious conditions suspected on the basis of clinical symptoms and peripheral blood findings.6 TABLE 16-1 Indications for Bone Marrow Examination Hypoplastic or aplastic anemia Idiosyncratic drug-induced marrow suppression Myelodysplastic syndromes such as refractory anemia Marrow necrosis secondary to tumor Marrow necrosis secondary to severe infection such as parvovirus B19 infection Immune versus amegakaryocytic thrombocytopenia Differentiation of megaloblastic, iron deficiency, sideroblastic, hemolytic, and blood loss anemia Estimation of storage iron to assess for iron deficiency Bone marrow specimen collection is a collaboration between a medical laboratory scientist (medical technologist) and a skilled specialty physician, often a pathologist or hematologist.7 Prior to bone marrow collection, the medical laboratory scientist collects peripheral blood for a complete blood count with blood film examination. During collection, the scientist assists the physician by managing the specimens and producing initial preparations for examination. The core biopsy specimen is particularly important for evaluating diseases that characteristically produce focal lesions, rather than diffuse involvement of the marrow. Hodgkin lymphoma, non-Hodgkin lymphoma, multiple myeloma, metastatic tumors, amyloid, and granulomas produce predominantly focal lesions. Granulomas, or granulomatous lesions, are cell accumulations that contain Langerhans cells—large, activated granular macrophages that look like epithelial cells. Granulomas signal chronic infection. The biopsy specimen also allows morphologic evaluation of bony spicules, which may reveal changes associated with hyperparathyroidism or Paget disease.8 Bone marrow collection sites include the following: • Posterior superior iliac crest (spine) of the pelvis (Figure 16-1). In both adults and children, this site provides adequate red marrow that is isolated from anatomic structures which are subject to injury. This site is used for both aspiration and core biopsy. • Anterior superior iliac crest (spine) of the pelvis. This site has the same advantages as the posterior superior iliac crest, but the cortical bone is thicker. This site may be preferred for a patient who can only lie supine. • Sternum, below the angle of Lewis at the second intercostal space. In adults, the sternum provides ample material for aspiration but is only 1 cm thick and cannot be used for core biopsy. It is possible for the physician to accidentally transfix the sternum and enter the pericardium within, damaging the heart or great vessels. • Anterior medial surface of the tibia in children younger than age 2. This site may be used only for aspiration. • Spinous process of the vertebrae, ribs, or other red marrow–containing bones. These locations are available but are rarely used unless they are the site of a suspicious lesion discovered on a radiograph. • Antiseptic solution and alcohol pads. • Local anesthetic injection, usually 1% lidocaine, not to exceed 20 mL per patient. • No. 11 scalpel blade for skin incision. • Disposable Jamshidi biopsy needle (Care Fusion; Figure 16-2) or Westerman-Jensen needle (Becton, Dickinson and Company, Franklin Lakes, N.J.; Figure 16-3). Both provide an obturator, core biopsy tool, and stylet. A Snarecoil biopsy needle also is available (Kendall Company, Mansfield, Mass.). The Snarecoil has a coil mechanism at the needle tip that allows for capture of the bone marrow specimen without needle redirection (Figure 16-4). • Disposable 14- to 18-gauge aspiration needle with obturator. Alternatively, the University of Illinois aspiration needle may be used for sternal puncture. The University of Illinois needle provides a flange that prevents penetration of the sternum to the pericardium. • Microscope slides or coverslips washed in 70% ethanol. • Petri dishes or shallow circular watch glasses. • Vials or test tubes with closures. • Anticoagulant liquid tripotassium ethylenediaminetetraacetic acid (K3EDTA). • Zenker fixative: potassium dichromate, mercuric chloride, sodium sulfate, and glacial acetic acid; B5 fixative: aqueous mercuric chloride and sodium acetate, or 10% neutral formalin. Because Zenker fixative and B5 contain toxic mercury, controlled disposal is required.
Bone Marrow Examination
Bone Marrow Anatomy and Architecture
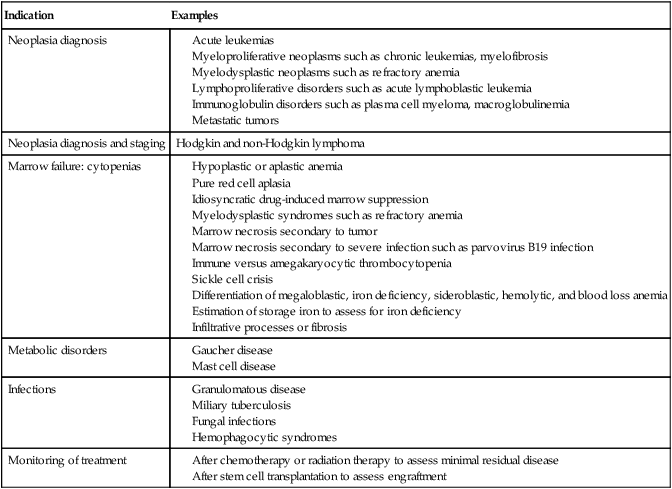
Indications for Bone Marrow Examination
Indication
Examples
Neoplasia diagnosis
Neoplasia diagnosis and staging
Hodgkin and non-Hodgkin lymphoma
Marrow failure: cytopenias
Metabolic disorders
Infections
Monitoring of treatment
Bone Marrow Specimen Collection Sites
Bone Marrow Aspiration and Biopsy
Preparation
Oncohema Key
Fastest Oncology & Hematology Insight Engine