Bone-Forming (Osteogenic) Lesions
Whether benign or malignant, bone-forming neoplasms are characterized by the formation of osteoid or mature bone directly by the tumor cells (27,28). Only one malignant tumor, osteosarcoma, is capable of doing this. The other bone-forming lesions are benign: osteoma, enostosis, osteoid osteoma, and osteoblastoma.
Benign Lesions
Osteoma
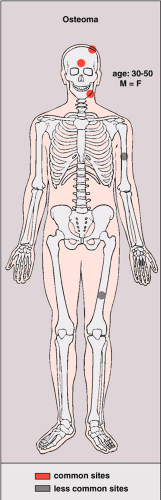
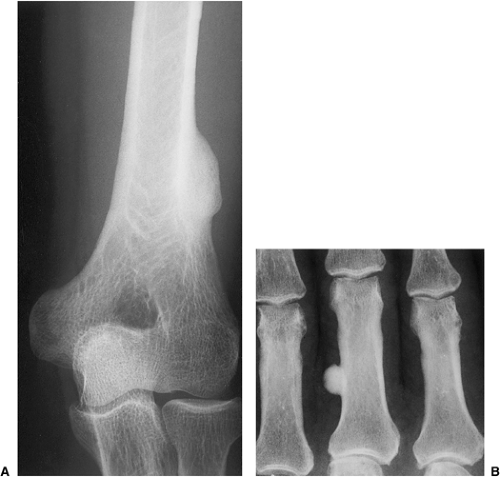
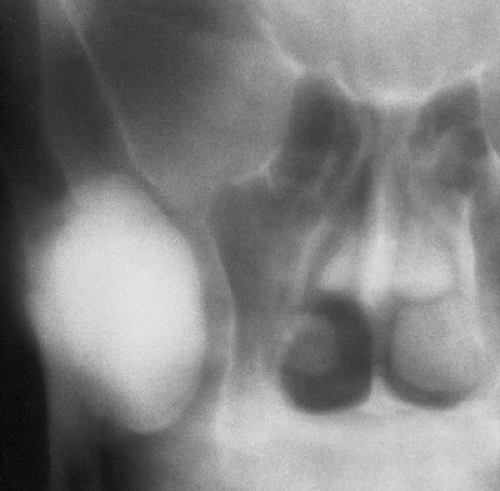
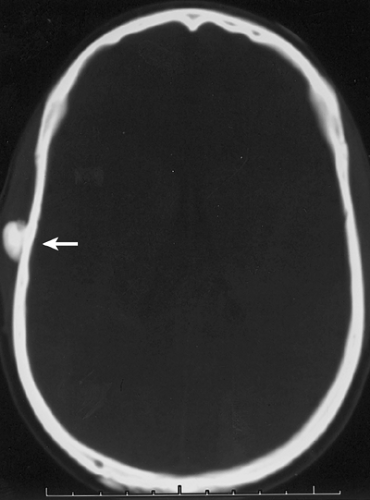
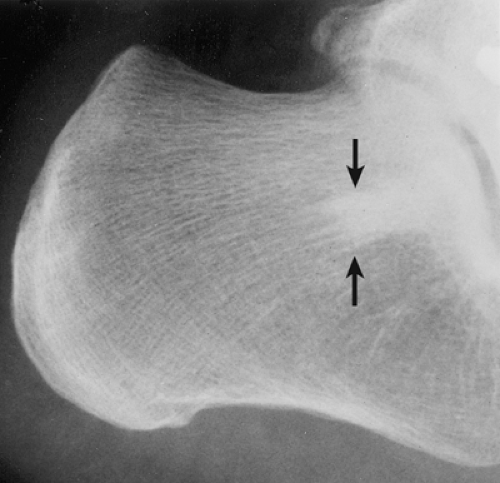
Osteoma is a benign, slow-growing lesion consisting of well-differentiated mature bone tissue with a predominantly lamellar structure, commonly arising in the frontal and ethmoid sinuses (approximately 75% of cases) (41). It may also occur on the surface of the outer table of the calvaria (“ivory exostosis”), the bones of the jaw (46), and, rarely, on the surface of the flat bones and the long and short tubular bones (2,30,32,36,49,50) (Fig. 2-1). Extracranial parosteal osteomas compose only 0.03% of biopsied primary bone lesions (34). Exceptionally, osteoma can exhibit endosteal extension (24).
Although not regarded as a neoplastic lesion by the World Health Organization (15), osteoma is included in this chapter because traditionally it has been regarded as a bone-forming lesion (5,13,16,34,52), and because of its importance in differential diagnosis of these lesions.
Clinical Presentation
Osteomas have been reported in patients from 10 to 79 years of age, with most in the fourth and fifth decades (13,46). Males and females are equally affected. The signs and symptoms associated with osteoma vary
depending on the size of the lesion and its location (37). Small lesions are usually asymptomatic and incidental findings. Large paranasal sinus tumors in particular may produce a variety of manifestations. Attached to the sinus wall and covered by the sinus mucoperiosteum (38), they often block the nasal ducts, causing mucoceles, sinusitis, nasal discharge, headache, and pain. At times a patient may lose the sense of smell (38). Occasionally, paranasal osteomas exhibit more dramatic features such as orbital or intracranial invasion (41). Tumors near the orbit can produce exophthalmos, double vision, and pressure on the optic nerve that may result in loss of vision. At other sites, they can erode the wall of the cranial fossa, perforate the dura, and compress the frontal lobe. Paranasal osteomas are usually discovered in the third and fourth decades, and the finding is most often incidental. One retrospective study involving 16,000 patients found a frequency of 0.4% for these tumors (11). They occur twice as frequently in males as in females and are believed to have a growth peak at the time of maximum skeletal development; they are only occasionally found before puberty (47).
depending on the size of the lesion and its location (37). Small lesions are usually asymptomatic and incidental findings. Large paranasal sinus tumors in particular may produce a variety of manifestations. Attached to the sinus wall and covered by the sinus mucoperiosteum (38), they often block the nasal ducts, causing mucoceles, sinusitis, nasal discharge, headache, and pain. At times a patient may lose the sense of smell (38). Occasionally, paranasal osteomas exhibit more dramatic features such as orbital or intracranial invasion (41). Tumors near the orbit can produce exophthalmos, double vision, and pressure on the optic nerve that may result in loss of vision. At other sites, they can erode the wall of the cranial fossa, perforate the dura, and compress the frontal lobe. Paranasal osteomas are usually discovered in the third and fourth decades, and the finding is most often incidental. One retrospective study involving 16,000 patients found a frequency of 0.4% for these tumors (11). They occur twice as frequently in males as in females and are believed to have a growth peak at the time of maximum skeletal development; they are only occasionally found before puberty (47).
Osteomas seldom develop in the long and short tubular bones (1,5,20,49) and are extremely rare in the flat bones (7,33,48). Tumors at these sites are known as parosteal osteomas. Like osteomas elsewhere, they are usually asymptomatic. Most parosteal lesions measure 1 to 4 cm in diameter, rarely reaching the dimensions reported by Baum et al. (16.5 × 4.2 cm) and Mirra et al. (6 × 5 × 5 cm) (1,33). Many long bone lesions are multiple.
It is worth noting the association of osteomas with the familial adenomatous polyposis syndrome (Gardner syndrome), an autosomal-dominant disorder with 25% new mutations, frequently seen in Mormons in Utah (10,18). It is caused by mutations in the APC gene, which maps to chromosome 5q21–q22 (3). In addition to bone lesions, the syndrome is marked by multiple cutaneous and subcutaneous lesions (e.g., sebaceous cysts, skin fibromas, and desmoid tumors) and intestinal, particularly colonic, polyposis (17). The bone tumors may precede the appearance of the intestinal polyps, which have a marked propensity to carcinomatous change (8,17). Osteomas have also been reported in association with tuberous sclerosis, an autosomal-dominant disorder affecting TSC1 (9q34) or TSC2 (16p13) gene, characterized by mental retardation, epilepsy, cutaneous hamartomas, and benign juvenile or inflammatory polyps of the colorectum (3,33). Finally, osteomas of the soft tissues (so-called osseous choristomas) are rare occurrences that have been described in the thigh, in the intraoral soft parts, and in the bronchi (12,29,42,45).
Imaging
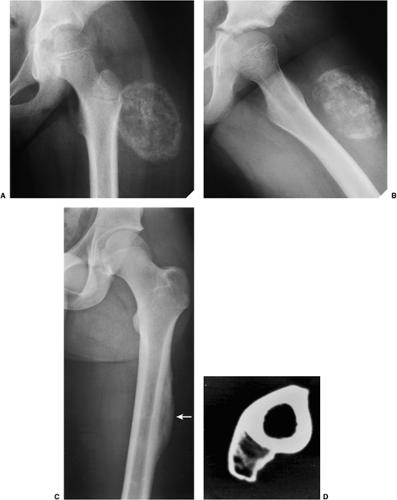
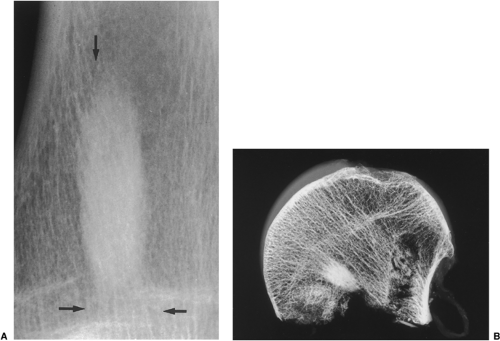
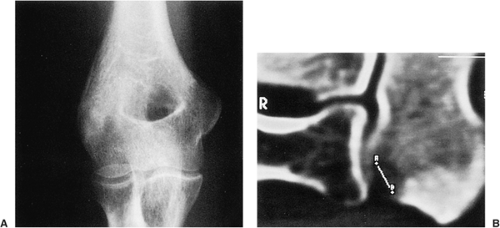
Conventional radiography is the basis for the diagnosis of osteoma. Radiographs typically show a dense, ivory-like sclerotic mass with sharply demarcated borders attached to the bone (Fig. 2-2). Conventional tomography (Fig. 2-3) and computed tomography (CT) (Fig. 2-4) are effective in demonstrating lack of
cortical invasion (50), which not infrequently is a feature of parosteal osteosarcoma, or lack of cortical and medullar continuity with a host bone, a feature of osteochondroma (see “Differential Diagnosis,” later). Magnetic resonance imaging (MRI) shows low signal intensity on both T1 and T2 sequences, consistent with cortical bone (50).
cortical invasion (50), which not infrequently is a feature of parosteal osteosarcoma, or lack of cortical and medullar continuity with a host bone, a feature of osteochondroma (see “Differential Diagnosis,” later). Magnetic resonance imaging (MRI) shows low signal intensity on both T1 and T2 sequences, consistent with cortical bone (50).
Histopathology
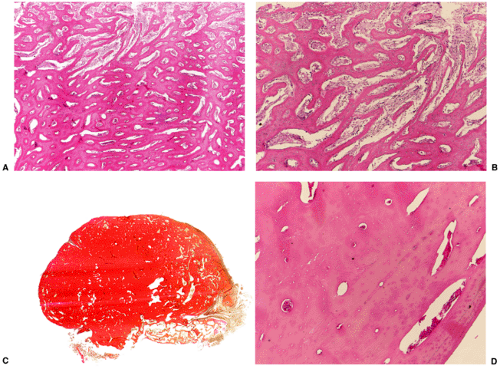
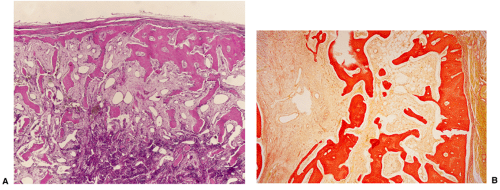
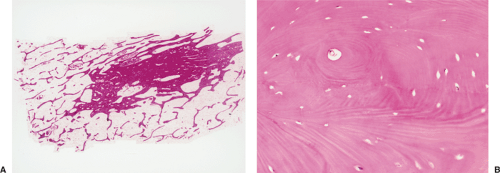
Histologically, two types of osteoma may be encountered: cancellous and compact (51). Most investigators consider the former to be the forerunner of the latter. The cancellous (or trabecular, or spongy) osteoma reveals a cancellous, trabecular architecture. Trabeculae are thin with fatty marrow present in the intertrabecular spaces (25) (Fig. 2-5A). They usually contain more woven bone and show evidence of active bone formation with transformation to lamellar bone (39). Woven bone consists of a more or less mature matrix with a plait of collagen fibers; it contains roundish osteocyte lacunae in high numbers per space unit. Lamellar bone is composed of narrow parallel layers of mature matrix with densely packed collagen fibers; it contains spindle-shaped osteocyte lacunae in smaller numbers than woven bone. Compact osteoma, on the other hand, consists of dense, compact, mature lamellar bone (9) (Figs. 2-5B, C). No Haversian systems are present and only occasionally marrow spaces are visible. A compact osteoma usually exhibits empty osteocyte lacunae in varying numbers, probably as a result of increased distance between the small marrow spaces and the cells that interferes with cell nourishment by diffusion.
Differential Diagnosis
Radiology
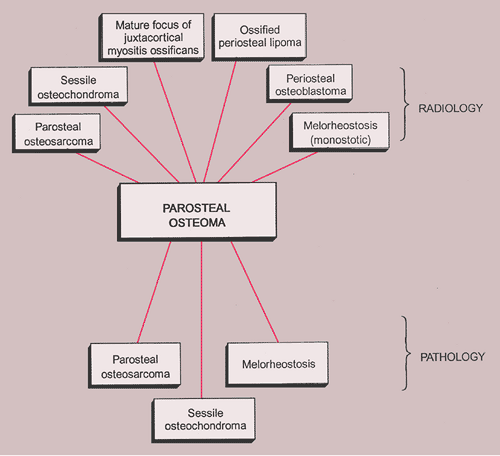
The radiologic differential diagnosis of solitary osteoma should include parosteal osteosarcoma, sessile osteochondroma, juxtacortical myositis ossificans, periosteal osteoblastoma, ossified parosteal lipoma, and focus of melorheostosis (21) (Fig. 2-6 and Table 2-1). Among these, parosteal osteosarcoma is the most important entity that needs to be excluded, and that may be a difficult task radiographically because both lesions appear as ivory-like masses attached to the bone’s surface (23) (Fig. 2-7). The keys to recognizing osteoma, however, are its usually exquisitely smooth borders and well-circumscribed, intensely homogeneous sclerotic appearance on radiographs. Parosteal osteosarcoma, in contrast, may show a zone of decreased density at the periphery and usually appears less dense and homogeneous than osteoma (53).
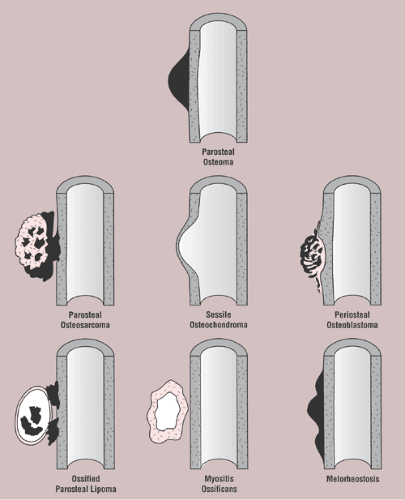 Figure 2-6 Schematic representation of differential possibilities of similarly appearing juxtacortical and cortical lesions. |
Table 2-1 Differential Diagnosis of Parosteal Osteoma | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
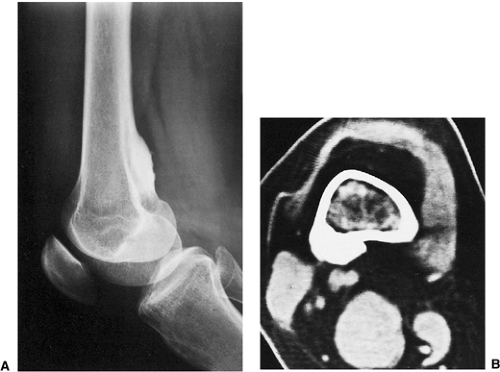 Figure 2-7 Parosteal osteosarcoma. A: Lateral radiograph shows ossific mass is attached to the posterior cortex of the distal femur. B: Computed tomography shows lack of invasion of bone marrow. |
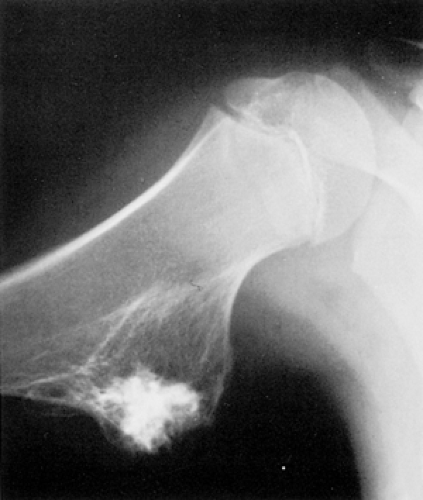 Figure 2-8 Sessile osteochondroma. Radiographic hallmarks include uninterrupted cortical and medullary continuity between the lesion and the host bone (humerus). |
Sessile osteochondroma can usually be identified by its characteristic radiographic features: the cortex of the lesion merges without interruption with the cortex of the host bone, and the cancellous portion is continuous with the host medullary cavity of the adjacent metaphysis or diaphysis (Fig. 2-8).
A well-matured focus of juxtacortical myositis ossificans may occasionally mimic osteoma. The radiographic hallmark of myositis ossificans is the so-called zonal phenomenon, characterized by a radiolucent area in the center of the lesion that indicates immature bone formation and a dense zone of mature ossification at the periphery. Often a thin radiolucent cleft separates the ossific mass from the adjacent cortex (Fig. 2-9A). Occasionally, however, a lesion (frequently, but not always, a mature one) may adhere to and fuse with the cortex, thus mimicking parosteal osteoma (Fig. 2-9B). In these instances CT may demonstrate the classic zonal phenomenon of the lesion (Fig. 2-9C).
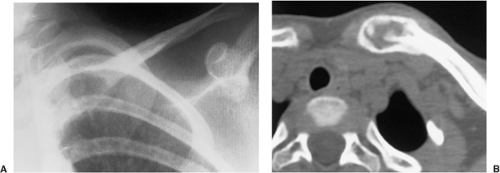
Periosteal osteoblastoma, an extremely rare bone-producing lesion (31,44), appears radiographically as a round-to-ovoid juxtacortical mass (19). Its less intense and less homogeneous density (Fig. 2-10) distinguishes it from osteoma.
Ossified parosteal (or periosteal) lipoma is a rare lesion, which in the majority of cases measures 5 to 7 cm at its greatest dimension (4,14,40,41,54). Pain is an unusual symptom; most patients give a history of a slow-growing, painless mass that has been present for many years (26). Consistent with fatty tumors generally, lobulation is the chief radiographic feature of the mass, which contains low-density fat in contrast to the surrounding muscle tissue that it displaces (35) (Fig. 2-11).
Melorheostosis is a rare form of mixed sclerosing dysplasia that on radiography appears as a long segment of cortical thickening (flowing hyperostosis), often resembling wax dripping down one side of a candle (6,22). A typical focus of monostotic melorheostosis usually exhibits both parosteal and endosteal involvement and the lesion commonly extends into the articular end of the bone (Fig. 2-12), features rarely present in a parosteal osteoma.
Pathology
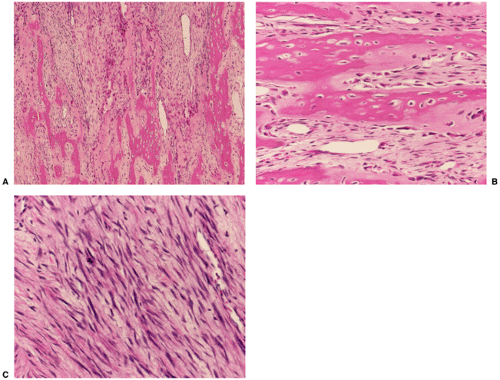
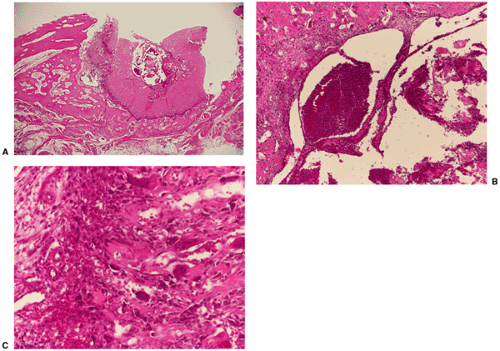
The difficulty in distinguishing between parosteal osteosarcoma and osteoma may extend to the histopathology. Conventional parosteal osteosarcoma is characterized by streamers of bone that appear woven to woven-lamellar and are produced by low-grade atypical, sometimes polar, cells of a fibroblastic stroma (33). Moderately cellular foci of stroma are noted, and nuclei may exhibit slight pleomorphism. Some lesions may exhibit a fairly cellular stroma containing neoplastic osteoid and bone, which clearly indicates malignancy. It may extend to and invade the underlying bone marrow. The heavily collagenous stroma of parosteal osteosarcoma is the key feature that distinguishes it from parosteal osteoma (51) (Fig. 2-13). In contrast, the presence of mature bone and the absence of an active fibrous stroma are the distinctive features of parosteal osteoma (1).
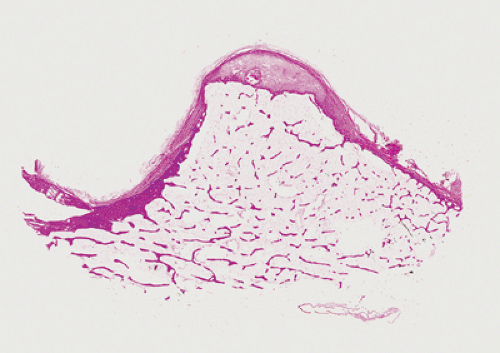
The microscopic features of sessile osteochondroma are similar to its radiographic appearance and are likewise diagnostic, with merging of the cortices and imperceptible blending of the spongiosa of the lesion and the host bone (Fig. 2-14). In addition, the spongiosa of sessile osteochondroma frequently contains remnants of calcified cartilage that was not completely replaced by bone during the growth phase of the lesion (33), a finding not present in osteoma. A cartilaginous cap of variable thickness on the surface of the osteochondroma is a common finding. Because a sessile-type lesion may lose this cap by ossification in late adulthood, it may be confused with osteoma; however, the latter is always attached to the intact cortex.
The remaining entities that may create difficulties in the differential diagnosis for the radiologist can easily be excluded by histopathologic characteristics. For example, microscopic examination of myositis ossificans circumscripta reveals a correlative histologic zonal phenomenon. In contrast to osteoma, the lesion consists of trabecular bone and marrow fat, not solid cortical bone (Fig. 2-15).
Small trabeculae of woven bone, numerous dilated capillaries, and exuberant hyperplasia of osteoblasts, osteoclasts, and, occasionally, fibroblasts are characteristic histopathologic findings of periosteal osteoblastoma (34) (Fig. 2-16). A thin shell of newly formed periosteal bone may cover the lesion (43).
Histologically, ossified parosteal lipoma can easily be distinguished from osteoma because it usually exhibits the typical features of lipoma, composed of adipose lobulated tumor tissue with mature fat cells and formation of woven bone trabeculae, and, at later stages, formation of lamellar bone with or without foci of necrosis and calcification (43).
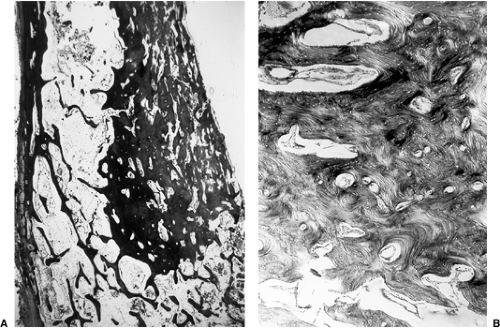
Also, melorheostosis can easily be distinguished histologically from an osteoma. The former is characterized by thickened trabeculae containing irregularly arranged Haversian canals surrounded by cellular
fibrous tissue (Fig. 2-17). Osteoblastic activity is usually present (6). This is in contrast to the thin trabeculae of the cancellous osteoma and the dense lamellar bone of the compact variant of this lesion.
fibrous tissue (Fig. 2-17). Osteoblastic activity is usually present (6). This is in contrast to the thin trabeculae of the cancellous osteoma and the dense lamellar bone of the compact variant of this lesion.
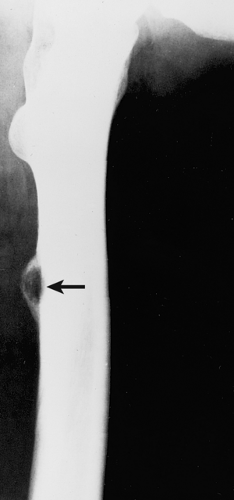 Figure 2-10 Periosteal osteoblastoma. Lateral radiograph of the femur shows a juxtacortical lesion with radiolucent center and more dense periosteal shell (arrow). |
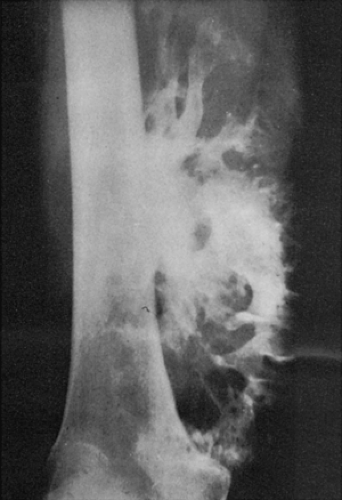 Figure 2-11 Ossified parosteal lipoma. Large ossific mass is attached to the medial cortex of the right femur. The radiolucent character of the fatty tissue (distal part of the tumor) is apparent. (Reprinted with permission from Greenfield GB, Arrington JA. Imaging of bone tumors. Philadelphia: JB Lippincott, 1995, Fig. 4-19.) |
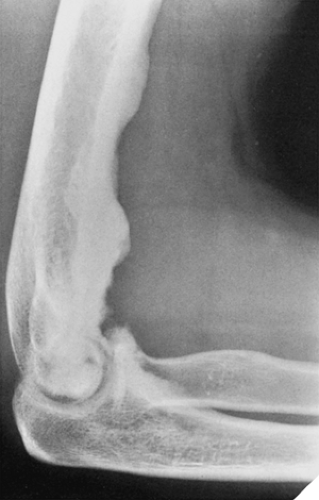 Figure 2-12 Forme fruste of melorheostosis. Lateral radiograph of the elbow shows a flowing hyperostosis of the anterior cortex of the distal humerus. Note the extension of the lesion into the joint. |
The radiologic and pathologic differential diagnosis of parosteal osteoma is depicted in Figure 2-18 and Table 2-1.
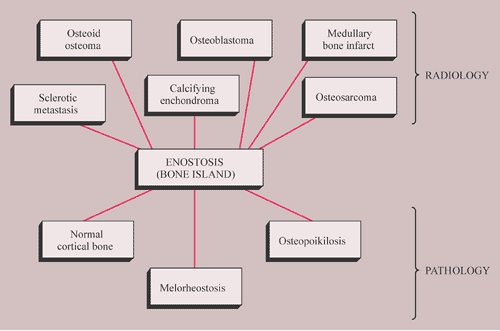
Enostosis (Bone Island)
Enostosis, known also as a bone island, is the endosteal compatriot of osteoma: a focus of cortical bone within cancellous (trabecular) bone (68). Although not in the true sense a “bone-forming” lesion, it is included in this chapter because it exhibits some morphologic features closely related to those of osteoma and some radiologic features similar to the other “true” bone-forming neoplasms (13,60).
Since its earliest descriptions in the literature, first by Stieda in 1905 and 7 years later by Fischer (68), enostosis has been variously named and defined. Stieda referred to the small, dense circumscribed shadows he observed inside the cancellous portion of short tubular bones and in the articular ends of long bones as “compact bone nuclei” (kompakte Knochenkerne). It was Fischer, however, who described these lesions as compact “islands” and emphasized their importance in differential diagnosis. Others have offered further names, including “calcified island in medullary bone” by Steel (68), “sclerotic bone island” by Meschan (72b), “focal sclerosis” by Caffey (59), and “end-osteoma” by Schmorl and Junghanns (77) (describing vertebral lesions). Definitions of this entity, as the names imply, have been similarly varied. Although some continue to classify enostosis among tumor-like conditions (58,60,62), Mirra (73) regards it as “misplaced, hamartomatous cortical bone.” In addition, recent investigations suggest that foci of mature compact bone within the spongiosa, which are characteristic of this condition, represent areas that failed to resorb during endochondral ossification (68,69). Probably of developmental or congenital origin, enostosis therefore represents an anomaly that appears closely related to osteopoikilosis (68). The importance of recognizing this benign lesion, which can be virtually diagnosed on the basis of its characteristic clinical and radiologic features, lies in the need to differentiate it from clinically more significant bone lesions, such as primary or metastatic tumors, when it manifests itself uncharacteristically by being very large (66,73) and showing activity on skeletal scintigraphy (69).
Clinical Presentation
Typically asymptomatic, a bone island is often an incidental finding on radiography performed for another purpose. It is discovered more commonly in adults than in children and shows no sexual predilection. The
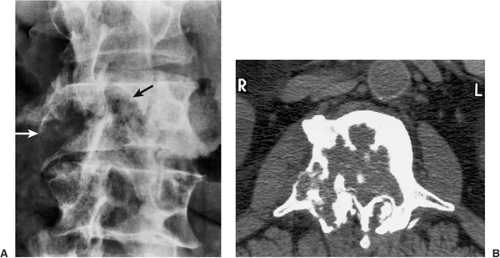
pelvis, the femur, and other long bones are preferential sites of involvement, although the lesion may be found anywhere in the skeleton, including the carpal and tarsal bones as well as the ribs (74). The spine is a rare site of involvement (57,77), accounting for only three (1.4%) of 209 bone islands reviewed by Onitsuka (75). These vertebral bone islands involved the thoracic and lumbar segments.
pelvis, the femur, and other long bones are preferential sites of involvement, although the lesion may be found anywhere in the skeleton, including the carpal and tarsal bones as well as the ribs (74). The spine is a rare site of involvement (57,77), accounting for only three (1.4%) of 209 bone islands reviewed by Onitsuka (75). These vertebral bone islands involved the thoracic and lumbar segments.
Imaging
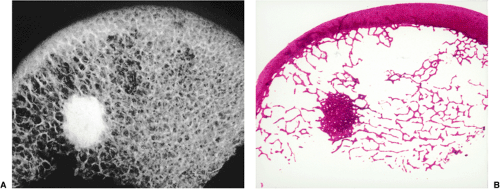
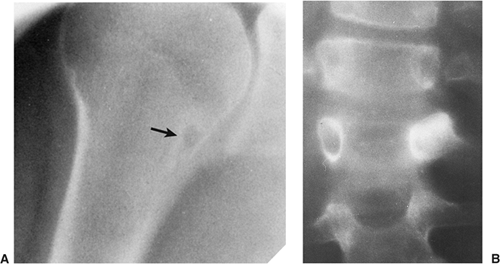
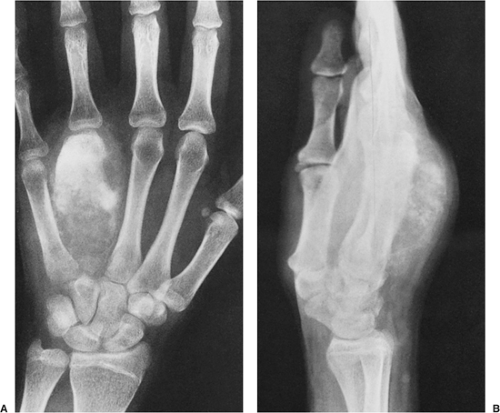
Regardless of its site or size, a bone island exhibits a consistent radiographic picture. The lesion appears as an ovoid, round, or oblong focus, homogeneously dense and sclerotic, in the cancellous bone (spongiosa). It is commonly oriented with the long axis of the bone parallel to the cortex. Highly distinctive for this lesion are radiating bony streaks, referred to as “thorny radiation” (70,71) or “pseudopodia” (67), aligned with the long axis of the host bone’s trabeculae, that merge with the trabeculae in a feathered or brush-like fashion (Figs. 2-19 and 2-20). In the great majority of cases, bone islands range in size from 1 mm to 2 cm (79). “Giant” bone islands, defined as lesions larger than 2 cm (65), have also been reported (Fig. 2-21). The largest lesion on record, described by Brien et al. (56) measured 10.5 × 4.8 × 4.0 cm and was located in the proximal femur. Another notable giant bone island reported by Park et al. (76) measured 10.0 × 1.7 × 1.0 cm, located in the tibia. An island reported by Gold et al. (65) measured 5 × 5 × 4.5 cm, also located in the tibia. Similarly, a bone island reported by Smith (79) measured 3.5 × 4 cm and was located in the ilium, and by Greenspan and Klein (66) measured 4 × 3 × 2.5 cm and was located in the distal femur. In addition to showing the same radiographic features as smaller lesions, giant bone islands may also give the “cumulus cloud” impression (73) (Fig. 2-22).
Bone islands usually do not show radiologic evidence of change in size over time. Nevertheless, several investigators have reported size changes in bone islands, some of which exhibited metabolic activity (55,64,71,72a,74,75). In the large series of patients who underwent multiple examinations described by Onitsuka (75), 44 of 138 bone islands (31.9%) showed changes in radiographic size over periods ranging from 3 to 23 years. After exclusion of 19 of these lesions because they had enlarged proportionally during adolescent growth, 21 of the remainder had increased in size and four had become smaller. Other evidence of growing bone islands is offered by Blank and Lieber (55), who reported six cases, by Ngan (74), who described
three cases and emphasized the possible confusion of such lesions with sclerotic metastases, and by Hoffman and Campbell (71), who reported the unstable appearance of a bone island. In the latter work the instability of a bone island was demonstrated by its disappearance and reappearance over a period of 6 years in a patient with hyperparathyroidism.
three cases and emphasized the possible confusion of such lesions with sclerotic metastases, and by Hoffman and Campbell (71), who reported the unstable appearance of a bone island. In the latter work the instability of a bone island was demonstrated by its disappearance and reappearance over a period of 6 years in a patient with hyperparathyroidism.
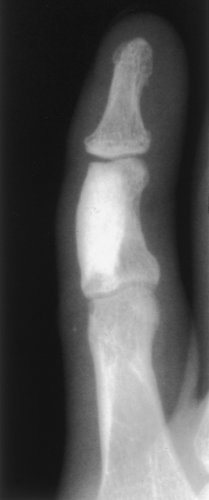 Figure 2-21 Giant bone island. A large sclerotic lesion occupies more than 50% of the middle phalanx of the small finger. |
On CT a bone island appears as a low-attenuation focus (63), exhibiting (as on radiography) its characteristic brush borders (Fig. 2-23A). Occasionally the lesion’s “pseudopodia” may show more rounded contours (Fig. 2-23-B). On all MRI pulse sequences a bone island exhibits the low signal intensity characteristics of cortical bone (Figs. 2-24 and 2-25).
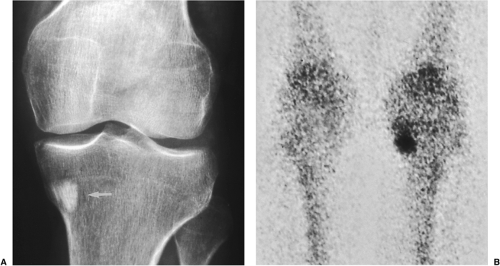
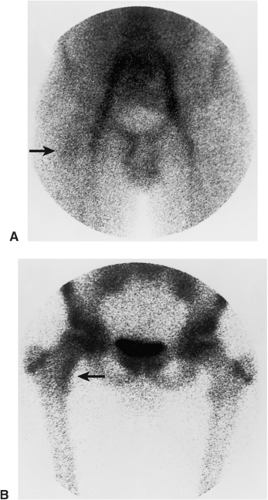
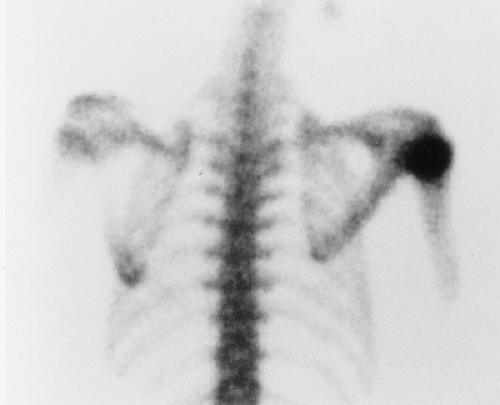
On skeletal scintigraphy a distinctive feature of bone islands is that they usually exhibit no activity. This is generally ascribed to the fact that their level of metabolic activity is about the same as that of the surrounding cancellous bone. Therefore, radionuclide imaging has been and continues to be the means of differentiating bone islands from more aggressive bone lesions. Nevertheless, reports in the literature of histologically confirmed bone islands that showed increased radiotracer uptake on bone scan (61,65,67,69,78) have raised a note of caution about the usefulness of scintigraphy in distinguishing a bone island from clinically more significant lesions (Fig. 2-26).
The phenomenon of scintigraphically active bone islands has prompted a number of explanations of the pathomechanism, which is still unclear. It has been linked to the large size of reported bone islands (78) and to their growth, based on the observation that uptake of the radiopharmaceutical agent is roughly proportional to the volume of a lesion. However, two large bone islands that were undetectable on bone scans, as reported by Hall et al. (70), indicate that a lesion’s size alone is not always a factor in this phenomenon. Others have pointed to a giant bone island’s histologic morphology and increased metabolic activity relative to the surrounding cancellous bone as the probable explanation for its striking radionuclide activity (65). Because tracer uptake implies greater metabolic activity and, usually, increased blood flow, it is logical to assume that a bone island will show increased activity if it becomes metabolically active. However, reports of radiographically documented growing bone islands (one with a positive and the other with a negative bone scan) that showed no histologic evidence of metabolic activity raise doubts about this pathomechanism (55,72).
A contribution by Greenspan et al. (67,69), correlating the radiologic and pathologic findings in six cases of bone island, suggested that the increased tracer uptake observed in two bone islands appeared to be directly related to the higher degree of bone remodeling and osteoblastic activity exhibited by these lesions in comparison with the scintigraphically “cold” lesions. On histologic examination, these “hot” bone islands were marked by a mixture of compact and trabecular bone,
with substantial amounts of woven bone. Vasculature was abundant, and osteoblastic activity with remodeling was significant. In contrast, the scintigraphically isoactive bone islands were composed solely of compact bone or mixed compact and trabecular bone, and showed negligible cellular activity and remodeling.
with substantial amounts of woven bone. Vasculature was abundant, and osteoblastic activity with remodeling was significant. In contrast, the scintigraphically isoactive bone islands were composed solely of compact bone or mixed compact and trabecular bone, and showed negligible cellular activity and remodeling.
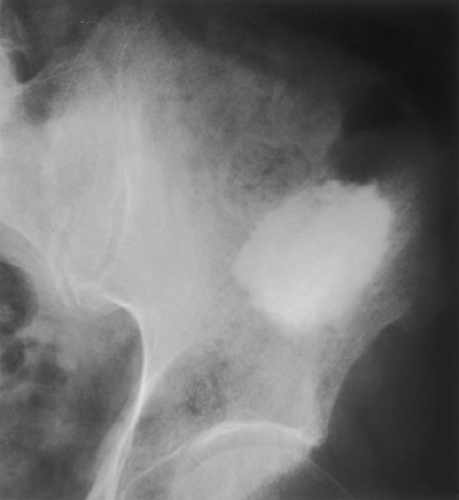 Figure 2-22 Giant bone island. Radiograph of the left ilium shows a large sclerotic lesion with the brush borders, assuming configuration of the “cumulus cloud.” |
Histopathology
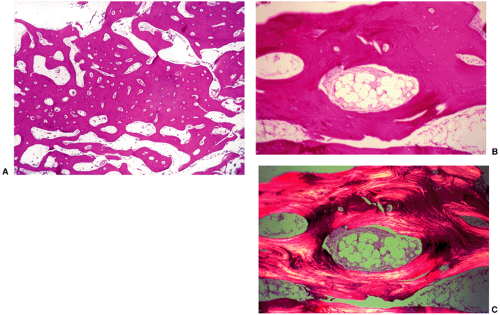
Microscopic examination of a bone island reveals a histologic picture that correlates with the radiographic findings. Enostosis is a focus of compact (cortical) bone in the spongiosa that shows thorn-like, thickened trabeculae radiating from the lesion and merging with the surrounding trabeculae of the host bone (58) (Fig. 2-27). Higher power magnification reveals mature lamellar configuration encircling Haversian systems of nutrient canals (Fig. 2-28). Bone islands occasionally contain foci of woven nonlamellar bone and, as reported in some scintigraphically active bone islands, they may consist of a mixture of lamellar and woven bone (65,67). Foci of osteoblastic and osteoclastic activity are rare (Fig. 2-29).
Differential Diagnosis
Radiology
In addition to its inclusion in the differential diagnosis of calcifying enchondroma and medullary bone infarct, as well as healing nonossifying fibroma, which may occasionally mimic it, an enostosis should be considered in the differential diagnosis of sclerotic medullary lesions that carry far more significant clinical implications than bone island, including osteoid osteoma, osteoblastoma, osteosarcoma, and sclerotic metastases. Scintigraphic activity in a lesion such as an enostosis that is generally believed to be “cold” on bone scan raises the possibility that a benign entity will be misdiagnosed as a more serious abnormality. The possibility also exists, regardless of activity on radionuclide imaging, that a tumor might be misdiagnosed as a bone island (73). Despite the continuing usefulness of bone scintigraphy in distinguishing bone islands from more aggressive lesions, finding activity in an otherwise suspected bone island cannot be a valid factor in the differential considerations (67).
In general, the presumptive diagnosis of a bone island can easily be made if the individual clinical and radiologic findings, together with follow-up examinations, are taken into consideration (56,80). The guides to the correct diagnosis are found in the lesion’s morphologic features as demonstrated on radiography, CT, and MRI, without reliance on scintigraphic findings. Thus, if a patient is found to have an asymptomatic isolated sclerotic bone lesion that exhibits the typical features of a bone island, with brush or feathered borders, the most likely diagnosis is an enostosis, whatever its size or its activity on bone scanning. Even the discovery of a sclerotic lesion in a patient with a
known neoplasm would strongly suggest a bone island if this lesion’s characteristic features are observed and the bone scan is normal. However, when a patient is symptomatic or the lesion appears “hot” on scintigraphy, misdiagnosis may occur in difficult cases. Such a circumstance demands careful observation with follow-up imaging studies. An open biopsy is in order if, as Mirra (73) suggested, the lesion’s growth exceeds 25% of its diameter within 6 months or 50% within 1 year.
known neoplasm would strongly suggest a bone island if this lesion’s characteristic features are observed and the bone scan is normal. However, when a patient is symptomatic or the lesion appears “hot” on scintigraphy, misdiagnosis may occur in difficult cases. Such a circumstance demands careful observation with follow-up imaging studies. An open biopsy is in order if, as Mirra (73) suggested, the lesion’s growth exceeds 25% of its diameter within 6 months or 50% within 1 year.
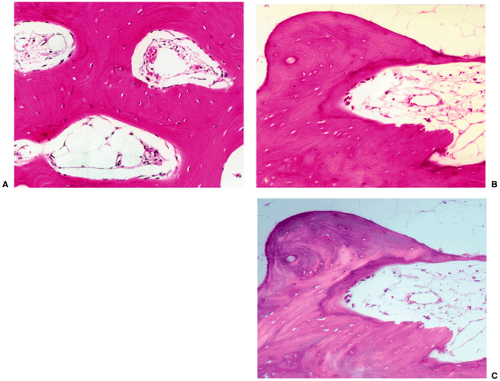 Figure 2-29 Bone island: histopathology. A: High-power photomicrograph (hematoxylin and eosin, original magnification ×230) of the bone island (same patient as in Fig. 2-26) shows large vascular spaces and osteoblastic cells along the bone surfaces. B: In another patient, a high-power photomicrograph (hematoxylin and eosin, original magnification ×250) shows osteoblastic activity and bone remodeling. C: Under polarized light the osteoblasts and parallel lamellae of mature bone surrounding the Haversian canal are well demonstrated. |
Pathology
The histologic differential diagnosis of enostosis rarely creates a serious problem, particularly when a pathologist is aware that the specimen has been obtained from the cancellous bone. Conversely, if only a minute fragment of bone island is submitted for examination, the microscopic picture may be indistinguishable from a normal cortical bone. Small biopsy specimen from the focus of melorheostosis may exhibit some histologic similarities to enostosis; however, correlation with radiographs clarifies the correct diagnosis. Likewise, the histologic differentiation of enostosis from osteopoikilosis (which in fact consists of multiple bone islands) must be based on imaging features.
The radiologic and pathologic differential diagnosis of enostosis is shown in Figure 2-30.
Osteoid Osteoma
Osteoid osteoma is a relatively common lesion of bone (4% of all primary bone tumors excluding myeloma, and 10% of all benign bone lesions), characterized by a nidus of osteoid tissue, that is usually less than 1 cm in diameter (93). The nidus, consisting of cellular, highly vascularized tissue that contains osteoid, can be entirely radiolucent or it may have a sclerotic center. In many instances the nidus is surrounded by a zone of reactive bone formation (98,114). Very rarely, an osteoid osteoma may present with more than one nidus. When two or more nidi are present within a single bone, the name multifocal osteoid osteoma is given, whereas multicentric osteoid osteoma refers to multiple nidi in different bones (107,134,138,143). Another variant of this
lesion has recently been reported, a “beaded” osteoid osteoma, which most likely represents transition between solitary and multifocal tumor (90).
lesion has recently been reported, a “beaded” osteoid osteoma, which most likely represents transition between solitary and multifocal tumor (90).
Clinical Presentation
Patients with osteoid osteoma almost invariably present with a complaint of nocturnal pain that may be severe enough to cause awakening (96,103). This is apparently due to the presence of prostaglandins that are demonstrated in the nidus but not in the surrounding bone (106,127,153). Recently, cyclooxygenase 1 and 2 (Cox-1 and Cox-2), apparently the source of these prostaglandins, have also been detected in osteoid osteoma by immunohistochemical methods (116,130). Salicylates, such as aspirin, are remarkably effective in relieving the pain, usually in less than half an hour. This classical presentation is typical in greater than 75% of patients and provides an important clue to the diagnosis (148). Local swelling and point tenderness are present in some patients (81,85,137). There may be also neurologic signs and symptoms, which can include muscle atrophy, a decrease in deep tendon reflex responses, and variable degrees of sensory loss (149). In most cases, osteoid osteoma arises in the age range of 10 to 35 years (89,121). There is a marked predominance in males, with a number of studies reporting a male-to-female ratio of 2:1 to 4:1 (34,93,100,113,114,115,119,120,121) (Fig. 2-31).
Osteoid osteoma can arise in virtually any bone. The long bones are preferentially affected (approximately 65% of the lesions occur in the long bones), particularly the femur and tibia [over 53% of all osteoid osteomas are located in these bones with the proximal femur being most frequently affected (91)], in which the lesions usually occur near the end of the shaft. Less commonly affected sites are the phalanges of hands and feet (21%), the vertebrae (9%), the humerus (122), and the scapula (88).
Depending on its location in the particular part of the bone, the lesion can be classified as cortical, medullary (cancellous), or subperiosteal. Osteoid osteomas can be further subclassified as extra- or intracapsular (intraarticular) (98,121). Recently, Kayser et al. (117) hypothesized that many osteoid osteomas arising in the tubular bones may in fact originate in a subperiosteal site and later appear as the intracortical lesions.
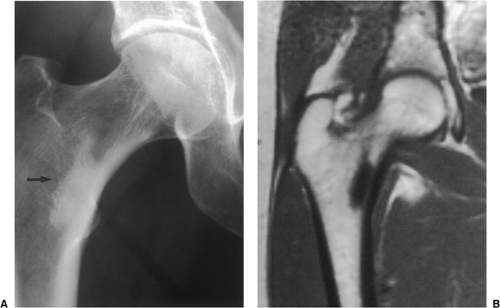
Intraarticular osteoid osteomas preferentially affect the hip (149). However, lesions in the elbow, foot, wrist, knee, and vertebral facet joints have also been reported (101,105). These tumors are usually characterized by nonspecific symptoms that are indicative of an inflammatory synovitis (82,144). Sclerosis may be lacking and the periosteal reaction is absent. On physical examination, a joint effusion and synovitis may be identified (82,92). Intracapsular lesions may also cause a precocious appearance of arthritis. This can provide an important diagnostic clue in cases when the history is typical of osteoid osteoma but the nidus has not been confirmed by radiography (132). If a lesion is located near the growth plate, particularly in a young child, it may cause accelerated bone growth.
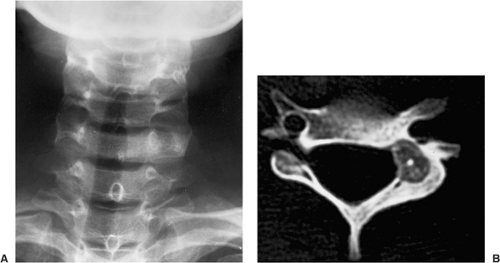
Osteoid osteoma involves the axial skeleton in 10% of cases (118,119,123,131,135). Osteoid osteoma of the spine most commonly involves the lumbar segment (59%). Less frequently affected are the cervical (27%), thoracic (12%), and sacral (2%) vertebrae (34,120,121,142). In patients with lesions of the neural arch painful
scoliosis often occurs (frequently as a result of a spasm), the concavity of the curvature being toward the side of the lesion (118).
scoliosis often occurs (frequently as a result of a spasm), the concavity of the curvature being toward the side of the lesion (118).
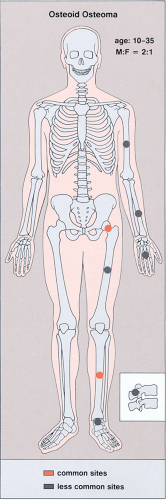 Figure 2-31 Osteoid osteoma: skeletal sites of predilection, peak age range, and male-to-female ratio. |
Imaging
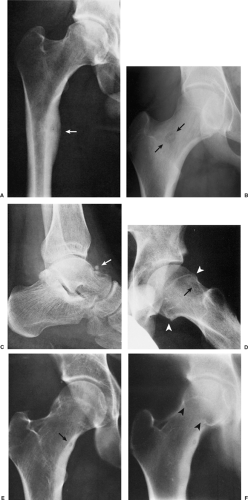
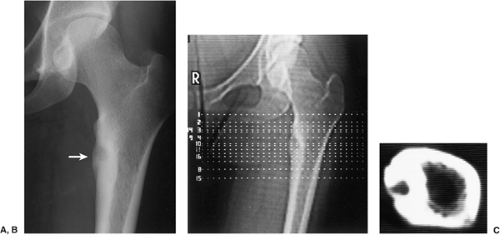
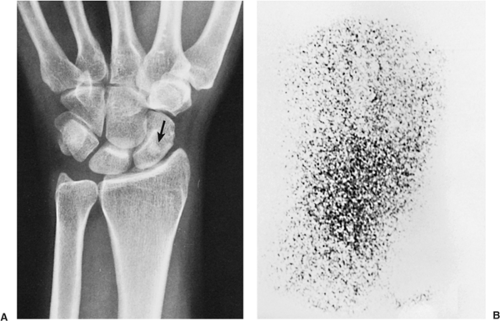
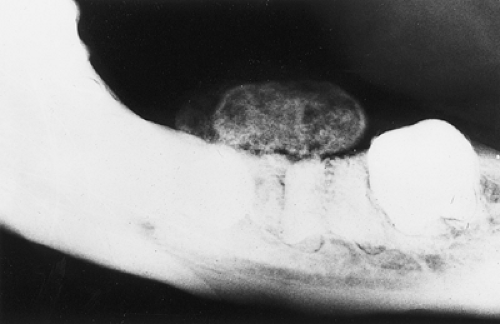
The probability of identifying osteoid osteoma by conventional radiography is dependent on the location of the lesion. Cortical lesions are usually characterized by a radiolucent nidus (representing the tumor itself) with a surrounding area of reactive sclerosis. A periosteal reaction may or may not be identified (Fig. 2-32A). An intramedullary nidus is less easily identified on radiography because very little or no reactive sclerosis is present (Fig. 2-32B). Occasionally, the nidus may exhibit a focus of central calcification (sclerotic center). The nidus of a subperiosteal lesion may be visualized either as a central radiolucent or sclerotic focus, with or without reactive sclerosis (Fig. 2-32C), or as a shaggy, crescent-like focus of periosteal reaction (120). The radiographic presentation of intracapsular lesions is frequently characterized by periarticular osteoporosis and in some instances by premature osteoarthritis (Fig. 2-32D) (132).
In some instances conventional tomography is used to evaluate osteoid osteoma, with better visualization of the nidus than can be obtained with conventional radiography (Fig. 2-33). It can also assist in confirming or excluding the presence of a sinus tract and is therefore useful when the differential diagnosis includes a possible bone abscess.
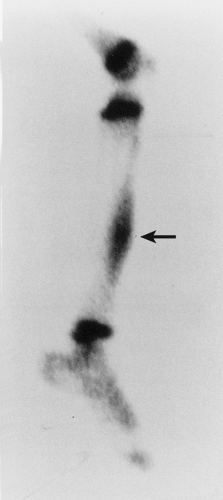
Skeletal scintigraphy is a highly sensitive method for detecting the nidus of osteoid osteoma (125,139,152). This modality can be particularly helpful in cases for which the symptoms are atypical and the initial radiographs appear normal (146). A three-phase radionuclide bone scan, using technetium-99 as a scanning agent, has been suggested (111), which can be especially valuable when intraarticular or intramedullary lesions are not clearly visualized by conventional radiography. Radionuclide tracer activity can be observed on both immediate and delayed images (Fig. 2-34). Osteoid osteoma is occasionally characterized by an interesting scintigraphic feature, the so-called double-density sign. This phenomenon is believed to be related to increased vascularity of the nidus (110,111). In agreement with the appearance of the lesion on radiography, the double-density sign is seen as a small focus of increased activity associated with the nidus; this focus is surrounded by a larger area of less intense activity, related to the reactive sclerosis that surrounds the nidus (Fig. 2-35). Identification of the double-density sign can help to differentiate an osteoid osteoma from a bone abscess.
Ultrasound can be helpful in diagnosis of intraarticular lesions (97), and Doppler duplex color technique can be used to localize the nidus (102).
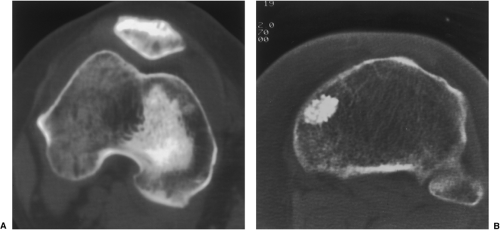
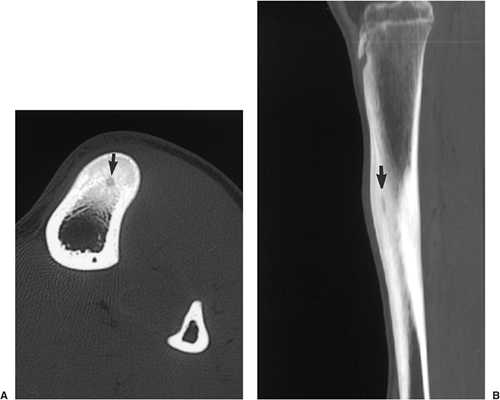
As the definite study for diagnosis of osteoid osteoma, CT is recommended. Not only can CT identify the lesion (151) but it can also accurately determine its
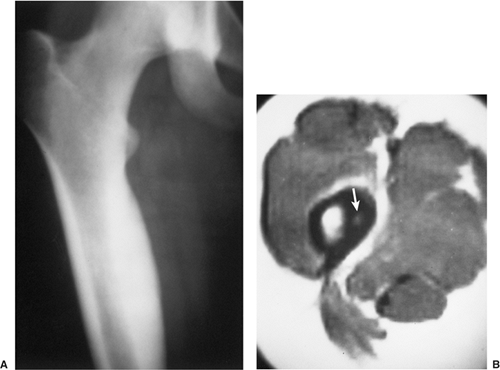
extent, thus enabling exact measurement of the size and location of the nidus (103,105,112,140,141) (Fig. 2-36). The nidus usually appears as a well-defined area of low attenuation, which is surrounded by a variably sized region of high attenuation reactive sclerosis (Fig. 2-37). To delineate the lesion, thin (preferably 2-mm) contiguous sections are most appropriate (Fig. 2-38). CT is particularly useful to define lesions that arise in the axial skeleton, whose complex anatomy is less clear on routine radiography and conventional tomography (145) (Fig. 2-39).
extent, thus enabling exact measurement of the size and location of the nidus (103,105,112,140,141) (Fig. 2-36). The nidus usually appears as a well-defined area of low attenuation, which is surrounded by a variably sized region of high attenuation reactive sclerosis (Fig. 2-37). To delineate the lesion, thin (preferably 2-mm) contiguous sections are most appropriate (Fig. 2-38). CT is particularly useful to define lesions that arise in the axial skeleton, whose complex anatomy is less clear on routine radiography and conventional tomography (145) (Fig. 2-39).
The suitability of MRI for detection of osteoid osteoma remains unclear and published reports have had mixed results (99,109,150,155,157). Goldman et al. (105) reported on four cases of intracapsular osteoid osteoma of the femoral neck, in which the lesions were evaluated with bone scintigraphy, CT, and MRI. Although in all cases abnormal findings were apparent in the MR images, the nidi could not be identified prospectively. On the basis of MRI findings of secondary bone marrow edema or synovitis, several incorrect diagnoses were made, which included Ewing sarcoma, osteonecrosis, stress fracture, and juvenile arthritis. In these cases, it is noteworthy that the correct diagnoses were made only after review of the radiographs and thin-section CT studies. Another report by Woods et al. (154) involved three patients with a highly unusual association of osteoid osteoma with a reactive soft tissue mass. In these cases, MRI studies might have led to confusion of osteoid osteoma with osteomyelitis or a malignant tumor. Moreover, in each case the nidus displayed different signal characteristics. In one case the intensity of signal was generally low on all pulse sequences but mild enhancement was seen after administration of gadolinium. In another case, the signal was of intermediate intensity and administration of gadolinium revealed inhomogeneous enhancement of the nidus. For the third case in which plain films showed the nidus to be intracortical, MRI could not identify the nidus distinctly. More recently, Davies et al. analyzing the MR imaging findings in 43 patients with osteoid osteoma concluded that reliance on MRI alone may lead to misdiagnosis (95). They suggested additional application of skeletal scintigraphy and CT in unclear cases, particularly when unexplained areas of bone marrow edema are encountered.
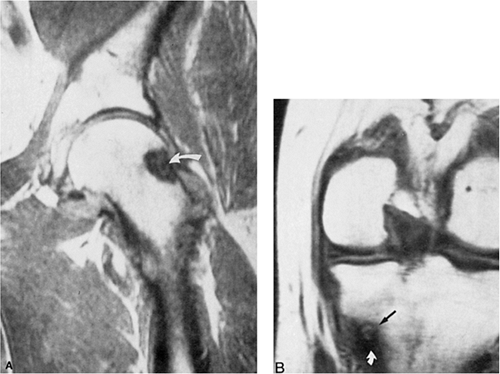
However, some reports do suggest the effectiveness of MRI for demonstrating the nidus of osteoid osteoma (84,104,156) (Figs. 2-40 and 2-41). Bell et al. (87) clearly demonstrated an intracortical nidus on MRI that had not been seen on scintigraphy, angiography, or CT scans. The nidus exhibits high signal intensity on T2-weighted sequences, most likely due to abnormal Cox-1 or Cox-2 levels (116). Recently, Liu et al. (126) advocated imaging of osteoid osteoma with dynamic gadolinium-enhanced MRI for greater conspicuity.
Histopathology
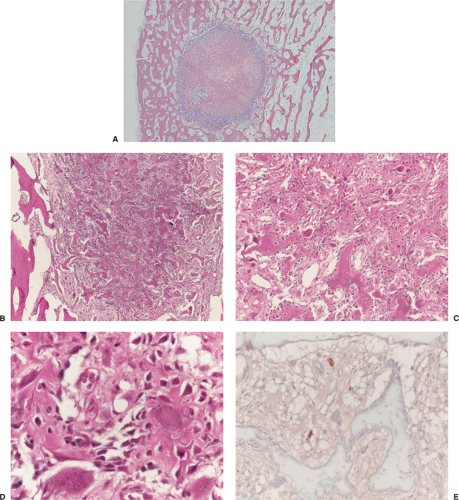
In lesions of osteoid osteoma, the nidus is small, well circumscribed, and self-limited. It is composed of osteoid tissue or mineralized immature woven bone (Figs. 2-42A–C). The osteoid matrix and bone form small and irregular trabeculae, with a thickness ranging from thin and delicate to broad and sclerotic (120). The trabeculae are surrounded by a highly vascular fibrous stroma that exhibits prominent osteoblastic and osteoclastic activity (Fig. 2-42D). The sclerosis surrounding the lesion is composed of dense bone that displays a variety of maturation patterns. From the ultrastructural viewpoint, the morphology of osteoblasts of osteoid osteoma is
generally similar to that of normal osteoblasts. However, these osteoblasts possess irregular, indented nuclei (indicative of high metabolic activity), glycogen particles, an abundance of fine intracytoplasmic fibers, and occasional iron-containing lysosomes (147). Moreover, some of these osteoblasts possess atypical mitochondria with a lobulated or “honeycomb” appearance. The areas of mineralized matrix exhibit the morphology of coarse woven bone. In two of the cases reported by Steiner (147), the osteoid contained, in addition to collagen, fine granular material, probably representing polysaccharides. Applying immunohistochemistry, nerve fibers positive for S-100, PGP 9.5 and/or neurofilament could be demonstrated in the fibrous zone and also in the nidus of osteoid osteoma, but not in other tumors including osteoblastoma (108,133). This finding in combination with prostaglandin and Cox-1/Cox-2 synthesis may explain the almost pathognomonic clinical presentation of this lesion.
generally similar to that of normal osteoblasts. However, these osteoblasts possess irregular, indented nuclei (indicative of high metabolic activity), glycogen particles, an abundance of fine intracytoplasmic fibers, and occasional iron-containing lysosomes (147). Moreover, some of these osteoblasts possess atypical mitochondria with a lobulated or “honeycomb” appearance. The areas of mineralized matrix exhibit the morphology of coarse woven bone. In two of the cases reported by Steiner (147), the osteoid contained, in addition to collagen, fine granular material, probably representing polysaccharides. Applying immunohistochemistry, nerve fibers positive for S-100, PGP 9.5 and/or neurofilament could be demonstrated in the fibrous zone and also in the nidus of osteoid osteoma, but not in other tumors including osteoblastoma (108,133). This finding in combination with prostaglandin and Cox-1/Cox-2 synthesis may explain the almost pathognomonic clinical presentation of this lesion.
Differential Diagnosis
Radiology
Even when a cortical lesion exhibits the classic radiographic appearance of osteoid osteoma, the differential diagnosis must consider an osteoblastoma (159,176), a cortical stress fracture, a focus of infection (124), and intracortical osteosarcoma (123). Only exceptionally osteoid osteoma may simulate an osteocartilaginous exostosis (128). The main differential factor to be considered in distinguishing osteoid osteoma from osteoblastoma is the size of the lesion. Generally, osteoblastomas are larger, exceeding a diameter of 2 cm (see discussion under heading “Osteoblastoma”). Periosteal reaction may be more prominent than encountered in osteoid osteomas. In stress fracture, the radiolucency is usually more linear than that of osteoid osteoma, running perpendicular to or at an angle to the cortex rather than parallel to it (Fig. 2-43). A cortical bone abscess may have a similar radiographic appearance to that of osteoid osteoma, but it can usually be differentiated by a linear, serpentine tract that extends away from the abscess cavity (Fig. 2-44). In some instances, intracortical osteoid osteoma must be differentiated from intracortical osteosarcoma, a rare bone-forming malignancy that arises solely within the cortex of bone and grossly involves neither the medullary cavity nor the soft tissues (123,129,136). On radiography, the latter tumor appears as a radiolucent focus within the cortex (femur or tibia), surrounded by zone of sclerosis. Occasionally, fluffy densities can be demonstrated in the radiolucent zone (129). The cortex at the site of the lesion may bulge slightly or may be thickened. The reported size of the lesion varies from 1.0 to 4.2 cm (83).
In intramedullary lesions, the differential must again consider an osteoblastoma, a bone abscess (Brodie abscess) and, in a lesion with calcified nidus, a bone island
(enostosis). Osteoblastoma, as mentioned previously, is a larger lesion than osteoid osteoma, and, in addition, exhibits considerably less peritumoral reactive sclerosis. A medullary bone abscess exhibits a central radiolucency very similar to that of osteoid osteoma, but, similarly to the cortical focus of infection, it can usually be differentiated by the serpentine tract that extends from the abscess cavity toward the nearest growth plate (Figs. 2-45 and 2-46). A bone island is characterized on radiography by the lesion’s brush-like borders, which blend with surrounding trabeculae in a pattern likened to “thorny radiation” or “pseudopodia” (67,68) (Fig. 2-47;
see also Figs. 2-19 and 2-20). In addition, bone islands usually exhibit no increased activity on radionuclide bone scan (69) (Fig. 2-48).
(enostosis). Osteoblastoma, as mentioned previously, is a larger lesion than osteoid osteoma, and, in addition, exhibits considerably less peritumoral reactive sclerosis. A medullary bone abscess exhibits a central radiolucency very similar to that of osteoid osteoma, but, similarly to the cortical focus of infection, it can usually be differentiated by the serpentine tract that extends from the abscess cavity toward the nearest growth plate (Figs. 2-45 and 2-46). A bone island is characterized on radiography by the lesion’s brush-like borders, which blend with surrounding trabeculae in a pattern likened to “thorny radiation” or “pseudopodia” (67,68) (Fig. 2-47;
see also Figs. 2-19 and 2-20). In addition, bone islands usually exhibit no increased activity on radionuclide bone scan (69) (Fig. 2-48).
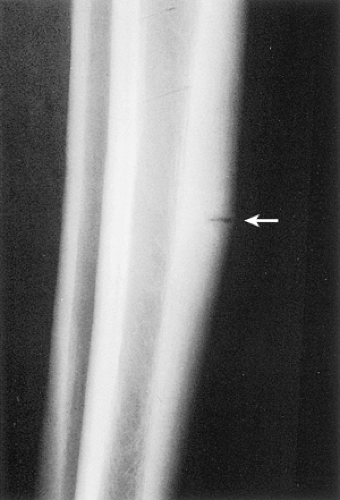 Figure 2-43 Cortical stress fracture. Lateral radiograph of the tibia shows the perpendicular direction of radiolucency to the long axis of the tibial cortex (arrow). |
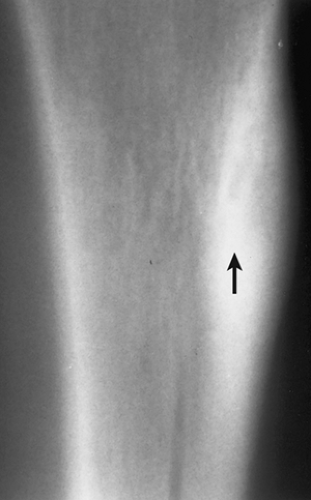 Figure 2-44 Cortical bone abscess. Lateral tomogram of the tibia shows a radiolucent, serpentine tract (arrow) that was originally misdiagnosed as osteoid osteoma. |
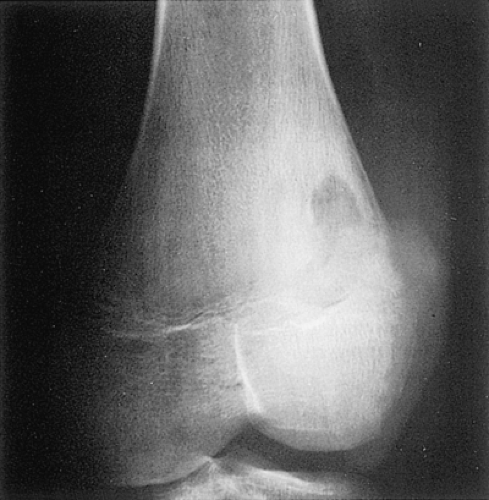 Figure 2-45 Medullary bone abscess. Anteroposterior radiograph of the distal femur shows a serpentine tract extending from an abscess cavity toward the growth plate. |
A schematic representation of differential possibilities of cortical and medullary osteoid osteoma is shown in Figure 2-49.
Pathology
Histopathologic differential diagnosis of osteoid osteoma should include primarily osteoblastoma and osteosarcoma because the histologic appearances of stress fractures, intracortical osteomyelitis, Brodie abscesses, and bone islands are unmistakably characteristic and do not create identification problems (Table 2-2).
Probably the most difficult task is to differentiate osteoid osteoma from osteoblastoma. In fact, some authorities (44) consider these entities to represent the same lesion in different stages of development because they show such remarkable histologic similarities. On the other hand, some investigators contend that osteoid production is usually greater in osteoblastoma than in osteoid osteoma and that the former lesion shows greater vascularity. Whereas in osteoid osteoma there seems to be a more organized structure with maturation of the nidus toward its periphery, in osteoblastoma the distribution of osteoid and trabecular bone has a less organized pattern: the whole lesion may be in the same stage of development (43). The difference in the size of the nidus (1.0 cm or less for osteoid osteoma and greater than 2 cm for osteoblastoma) may be an artificial division because osteoblastoma in the early stage of development has to be smaller than 2 cm. In the spine that division may be even more artificial, because the lesions of osteoblastoma are often smaller than 2 cm. The presence of more or less marked perilesional sclerosis in osteoid osteoma may allow this rather arbitrary differentiation.
However, genetic analysis revealed chromosomal alterations involving chromosome 22 in osteoid osteoma that were different from alterations observed in osteoblastomas (86,193).
However, genetic analysis revealed chromosomal alterations involving chromosome 22 in osteoid osteoma that were different from alterations observed in osteoblastomas (86,193).
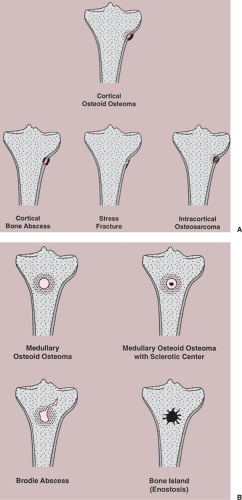 Figure 2-49 Schematic representation of differential possibilities of (A) cortical and (B) medullary osteoid osteoma. |
Table 2-2 Differential Diagnosis of Osteoid Osteoma | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Histologic differentiation of osteoid osteoma from osteosarcoma (mainly from the intracortical variant) is less challenging. The latter tumor is rich in osteoid and woven bone, with minimal anaplasia due to so-called normalization of nuclei. Trapping of host cortical lamellar bone within the tumor, however, is an indicator of malignancy (129).
Differentiation of osteoid osteoma from a bone island is not difficult. The histopathologic features of bone island are characteristic: a dense, sclerotic focus of compact bone within the cancellous bone exhibits thickened peripheral trabeculae blending with the trabeculae of the spongiosa. In addition, the lesion shows parallel or concentric bands of mature compact lamellar bone, and marrow spaces having the appearance of Haversian canals (67,68) (Fig. 2-50; see also Fig. 2-28).
The radiologic and pathologic differential diagnosis of osteoid osteoma is depicted in Figure 2-51 and Table 2-2.
Osteoblastoma
Osteoblastoma accounts for approximately 1% of all primary bone tumors and 3% of all benign bone lesions (176,177). Jaffe and Mayer are credited with identifying it as an entity in 1932 (178). Later it was described as “giant osteoid osteoma,” underscoring its close histologic resemblance to osteoid osteoma and its larger size (167). The lesion is often more than 2 cm in diameter (177). Lichtenstein termed it an “osteogenic fibroma of bone,” subsequently changing the name to “benign osteoblastoma” (30,31,182). The histologic similarities between osteoid osteoma and osteoblastoma are striking, and differentiation is often difficult if not impossible (163). Some authors subscribe to the concept that they represent different clinical expressions of the same pathologic process (44,170). In most of the world literature, however, osteoid osteoma and osteoblastoma are considered separate and distinct clinical entities, mainly because of their different clinical presentations and different radiologic characteristics. Their natural histories also differ: whereas osteoid osteoma tends toward regression, osteoblastoma tends toward progression and even malignant transformation (113) (although the possibility of the latter event remains controversial). Genetic findings are also in favor of separating these lesions (94,192).
Clinical Presentation
Like osteoid osteoma, osteoblastomas are most often found in patients in the first to third decades with the peak incidence in the second decade (184); “aggressive” tumors are usually seen in an older age group (average age 33 years) (180,187). The male-to-female ratio appears to be approximately 2:1 (122,175). The long tubular bones are frequently affected, but the lesion shows a distinct predilection for the axial skeleton in general and the vertebral column in particular (13,34,164,180,181) (Fig. 2-52). Approximately 30% to 40% are localized in the spine (131), more or less equally in the cervical, thoracic, and lumbar segments, mainly in the posterior elements including the arch and spinous processes (186). In one series of 123 tumors, 39 (32%) were located in the spine and 23 (19%) in the jaw; the remaining 61 tumors were distributed throughout the appendicular skeleton with a predilection for the femur and tibia (187). Periosteal osteoblastomas have occasionally been reported (190), but multifocal lesions are quite rare (158).
The clinical presentation of osteoblastoma is different from that of osteoid osteoma (159,176). Some patients
are asymptomatic, and the lesion is found incidentally (183). Symptomatic patients in one study most often experienced a dull, localized pain, with a median duration of symptoms of 6 months before they presented for treatment. The pain very rarely interfered with sleep. Although the use of salicylates for pain relief is not mentioned in the study by Kroon and Schurmans (180), the pain of osteoblastoma does not appear to be as readily relieved by salicylates as the pain in osteoid osteoma. Tenderness at the tumor site is a consistent physical finding (186), and swelling may be present, particularly when the lesion is near the surface (180).
are asymptomatic, and the lesion is found incidentally (183). Symptomatic patients in one study most often experienced a dull, localized pain, with a median duration of symptoms of 6 months before they presented for treatment. The pain very rarely interfered with sleep. Although the use of salicylates for pain relief is not mentioned in the study by Kroon and Schurmans (180), the pain of osteoblastoma does not appear to be as readily relieved by salicylates as the pain in osteoid osteoma. Tenderness at the tumor site is a consistent physical finding (186), and swelling may be present, particularly when the lesion is near the surface (180).
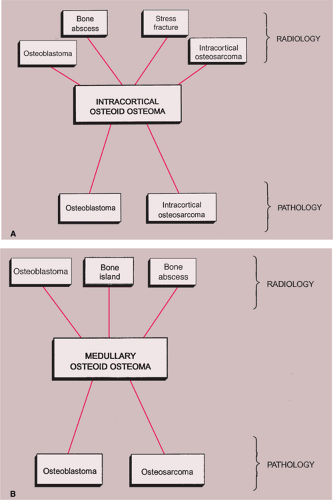 Figure 2-51 A: Differential diagnosis of intracortical osteoid osteoma. B: Differential diagnosis of medullary osteoid osteoma. |
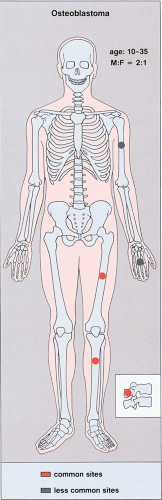 Figure 2-52 Osteoblastoma: skeletal sites of predilection, peak age range, and male-to-female ratio. |
Patients with lumbar vertebral lesions will very commonly present with scoliosis. Scoliosis also commonly occurs in patients whose tumors arose in the ribs (169,172). Spinal lesions in particular may cause neurologic deficits ranging from muscle weakness to paraplegia.
Toxic osteoblastoma, a rare variant of this tumor, has recently been recognized (166). It is associated with systemic manifestations, including diffuse periostitis of multiple bones, fever, and weight loss (191).
Osteoblastomas rarely extend into the soft tissues and do not metastasize, although they do tend to recur locally.
Imaging
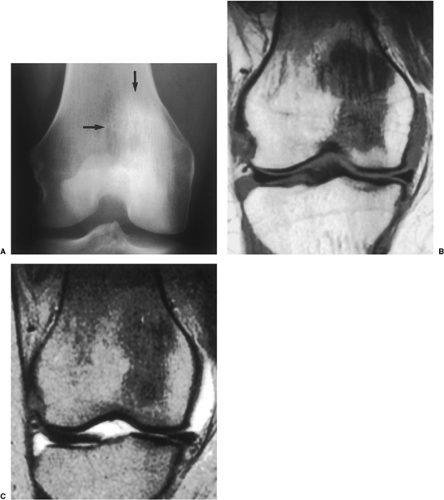
Conventional radiography is the basis for the diagnosis of osteoblastoma. In a review of the spectrum of osteoblastoma in 123 patients by McLeod et al. (187), most tumors (75%) were either spherical or slightly oval, with the remainder being longitudinal; the tumor margin was well defined (83%). In half of the appendicular tumors, there was considerable reactive sclerosis; 13% of these tumors, however, had only a sclerotic rim, and 37% showed no surrounding sclerosis. The interior of the lesions was most often radiolucent (64%), whereas various degrees of ossification were present in the remaining cases. The authors speculated that lesions tend to be radiolucent when they are younger, ossifying as they mature. In the great majority of tumors, the cortex was either perfectly normal or intact, although expanded and thinned (75%); the cortical expansion was usually eccentric. Destruction or penetration of the cortex suggesting malignancy was present in a minority (20%) of tumors. The great majority of osteoblastomas showed periosteal new bone formation, which in some cases was extremely prominent. The periosteal reaction was predominantly benign and of the solid type (86%), whereas the remainder showed spiculation or multilamination, suggesting a more aggressive lesion.
In a review of the range of manifestations of osteoblastoma by Marsh et al. (186), the majority of tumors showed a shell of reactive bone separating the tumor from the surrounding normal bone. Osteoblastomas of the spongy bone of the spine, ilium, or talus exhibited less prominent reactive bone formation, and those arising in the cortex more pronounced reactive bone, than is seen in osteoid osteoma.
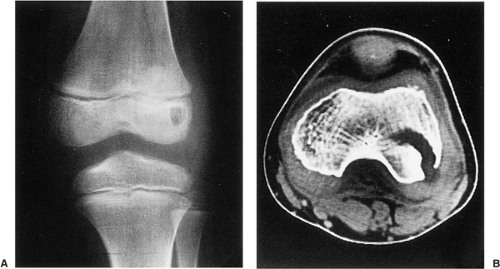
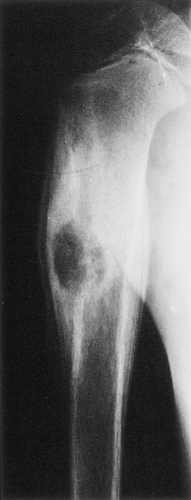
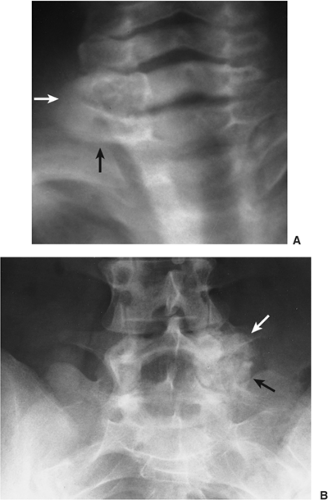
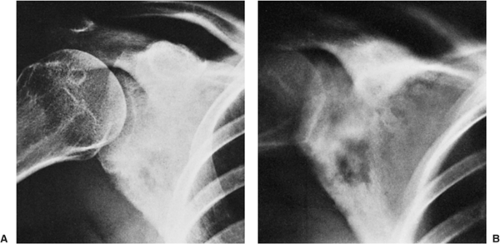
Four distinctive types of osteoblastomas can be identified, based on the radiographic findings (23). (a) The lesion may be almost identical to osteoid osteoma, although much larger (usually >2 cm in diameter). This type occasionally exhibits less reactive sclerosis than osteoid osteoma, but the periosteal reaction is more prominent. (Fig. 2-53). (b) Osteoblastoma may also appear as a blow-out expansive lesion similar to an aneurysmal bone cyst, with small radiopacities in the center. This pattern is particularly common in tumors involving the spine (Fig. 2-54). (c) The tumor may occasionally appear as an aggressive lesion, simulating a malignant neoplasm (Fig. 2-55). Many of the so-called aggressive osteoblastomas
belong to this group. (d) Exceptionally rare, osteoblastoma is juxtacortical (periosteal) in location, as described in 6 of 42 tumors reported by Schajowicz and Lemos (44) and in 2 of 20 tumors reported by Lichtenstein and Sawyer (31). These lesions generally lacked perifocal bone sclerosis and in addition, a thin shell of newly formed periosteal bone was noted covering the lesion (19,34,44) (Fig. 2-56; see also Fig. 2-10).
belong to this group. (d) Exceptionally rare, osteoblastoma is juxtacortical (periosteal) in location, as described in 6 of 42 tumors reported by Schajowicz and Lemos (44) and in 2 of 20 tumors reported by Lichtenstein and Sawyer (31). These lesions generally lacked perifocal bone sclerosis and in addition, a thin shell of newly formed periosteal bone was noted covering the lesion (19,34,44) (Fig. 2-56; see also Fig. 2-10).
Conventional tomography, although nowadays rarely used, may be valuable for determining the extent of the tumor and whether and to what degree intralesional bone formation has occurred. It is particularly useful for spinal lesions, which may be difficult to interpret on routine radiographs. Tomography may also help gauge the size of the nidus, thus facilitating the distinction from osteoid osteoma (Fig. 2-57).
Skeletal scintigraphy invariably demonstrates intense focal accumulation of bone-seeking radiopharmaceutical agents (Fig. 2-58). Angiography is infrequently performed as part of the evaluation of osteoblastoma because of its questionable importance as a preoperative assessment tool (180).
CT, as in osteoid osteoma, is the most important imaging modality for facilitating the diagnosis of osteoblastoma and demonstrating its exact size and location (174) (Fig. 2-59). In addition, areas of calcifications and ossifications within the lesion (Fig. 2-60), as well as cortical destruction and soft tissue extension, are well delineated on CT sections (180). CT is particularly helpful in working up lesions of the spine (159) (Fig. 2-61).
Very few reports on the usefulness of MRI in the diagnosis and evaluation of osteoblastoma can be found in the literature (180). The strength of MRI would appear to be similar to that of CT, namely, defining the extent of bone and soft tissue involvement. Most osteoblastomas demonstrate signal intensity patterns on MR scans
similar to those of the majority of bone neoplasms: spin-echo T1-weighted images show a low to intermediate intensity signal, whereas with T2-weighting signal intensity is intermediate to high. More sclerotic lesions are of low signal intensity on all pulse sequences (Fig. 2-62). MRI effectively reveals peritumoral edema in the adjacent marrow and in soft tissues. A recent report by Crim et al. (165) of a 19-year-old man with osteoblastoma described an unusual “flare phenomenon” that caused a misleading appearance on MR scans, simulating a malignant process such as lymphoma or Ewing sarcoma. The phenomenon represented a widespread inflammatory response to the lesion that led to diffuse, reactive inflammatory infiltrates in two vertebrae, the adjacent ribs, and the paraspinal soft tissues. It is noteworthy that only CT-myelography was diagnostic in this case.
similar to those of the majority of bone neoplasms: spin-echo T1-weighted images show a low to intermediate intensity signal, whereas with T2-weighting signal intensity is intermediate to high. More sclerotic lesions are of low signal intensity on all pulse sequences (Fig. 2-62). MRI effectively reveals peritumoral edema in the adjacent marrow and in soft tissues. A recent report by Crim et al. (165) of a 19-year-old man with osteoblastoma described an unusual “flare phenomenon” that caused a misleading appearance on MR scans, simulating a malignant process such as lymphoma or Ewing sarcoma. The phenomenon represented a widespread inflammatory response to the lesion that led to diffuse, reactive inflammatory infiltrates in two vertebrae, the adjacent ribs, and the paraspinal soft tissues. It is noteworthy that only CT-myelography was diagnostic in this case.
Histopathology
Osteoblastoma is marked by a very active new formation of osteoid and immature bone trabeculae produced by compact masses of large osteoblasts (44). Osteoid production is generally greater in osteoblastoma than in osteoid osteoma, and the lesion is more vascularized (185) (Fig. 2-63). Furthermore, osteoblastoma exhibits a less organized pattern of osteoid and trabecular bone distribution than the nidus of osteoid osteoma, which invariably appears as a circumscribed, organized structure maturing toward its periphery; in effect, the lesion of osteoblastoma is at the same stage of development throughout (44). Very rarely, cartilaginous matrix may
be present (161). Occasionally, spindle-shaped hyperchromatic cells with uniform nuclei and irregular eosinophilic cytoplasm may be interdispersed among bony trabeculae (173). In contrast to most forms of osteosarcoma, osteoblastomas characteristically show variable numbers of osteoclasts at the surfaces of the bone trabeculae and seams of osteoblasts rimming the periphery of the lesion (Figs. 2-64A–C). In addition, they do not infiltrate or permeate the normal trabeculae at the interface with the lesion, as occurs in osteosarcoma (34), but usually are separated from them by a narrow but distinct layer of bone-free fibrous tissue. Some atypical osteoblastomas may, however, closely resemble an osteosarcoma (Fig. 2-64D).
be present (161). Occasionally, spindle-shaped hyperchromatic cells with uniform nuclei and irregular eosinophilic cytoplasm may be interdispersed among bony trabeculae (173). In contrast to most forms of osteosarcoma, osteoblastomas characteristically show variable numbers of osteoclasts at the surfaces of the bone trabeculae and seams of osteoblasts rimming the periphery of the lesion (Figs. 2-64A–C). In addition, they do not infiltrate or permeate the normal trabeculae at the interface with the lesion, as occurs in osteosarcoma (34), but usually are separated from them by a narrow but distinct layer of bone-free fibrous tissue. Some atypical osteoblastomas may, however, closely resemble an osteosarcoma (Fig. 2-64D).
Some osteoblastomas contain “epithelioid” osteoblasts, twice the size of ordinary osteoblasts (171). The cells are rounded and have large nuclei containing one or more prominent nucleoli; their cytoplasm is usually abundant. Bone trabeculae are wider and more irregular than in conventional osteoblastoma, and cement lines are usually absent (179) (Fig. 2-65). These variants have been denoted as “aggressive” osteoblastomas. Della Rocca and Huvos (168) stated recently that a clinically aggressive behavior of osteoblastomas is not correlated with histologic qualities but with a localization within the skeleton that is more difficult to approach surgically. Schajowicz and Lemos (195) described eight patients with “malignant” osteoblastoma whose tumors
appear to be similar to “aggressive osteoblastoma.” They noted on microscopic examination more frequent atypical mitoses and increased numbers of osteoclast-like giant cells. In addition, there were bone spicules of regular shape (dark blue staining with hematoxylin), closely resembling those observed in osteosarcoma. Another pseudomalignant or pseudoanaplastic variant of osteoblastoma contains bizarre multinucleated cells completely devoid of mitotic activity (160,187,189).
appear to be similar to “aggressive osteoblastoma.” They noted on microscopic examination more frequent atypical mitoses and increased numbers of osteoclast-like giant cells. In addition, there were bone spicules of regular shape (dark blue staining with hematoxylin), closely resembling those observed in osteosarcoma. Another pseudomalignant or pseudoanaplastic variant of osteoblastoma contains bizarre multinucleated cells completely devoid of mitotic activity (160,187,189).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree