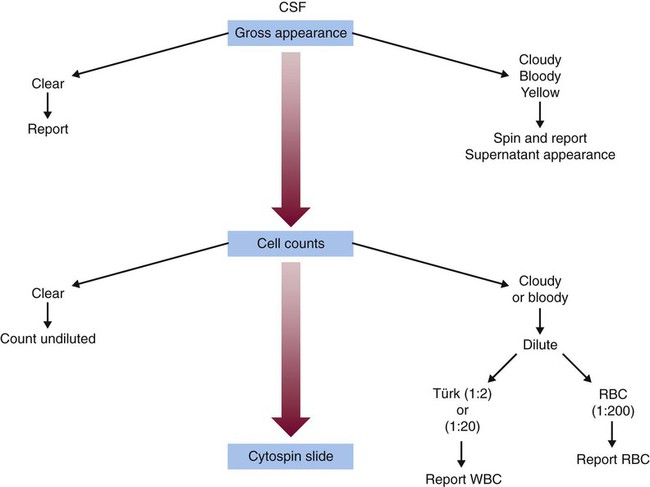
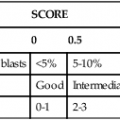
After completion of this chapter, the reader will be able to: 1. Describe the method for performing cell counts on body fluids. 2. Given a description of a body fluid for cell counting, choose the appropriate diluting fluid, select a counting area, and calculate and correct (if necessary) the counts. 3. Using the proper terminology, discuss the gross appearance of body fluids, including its significance and its practical use in determining cell count dilutions. 4. Discuss the advantages and disadvantages of cytocentrifuge preparations. 5. Differentiate between traumatic spinal tap and cerebral hemorrhage on the basis of cell counts and appearance of uncentrifuged and centrifuged specimens. 6. Identify from written descriptions normal cells found in cerebrospinal, serous, and synovial fluids. 7. Describe the characteristics of benign versus malignant cells in body fluids and recognize written descriptions of each. 8. Differentiate exudates and transudates based on formation (cause), specific gravity, protein concentration, appearance, and cell concentration. 9. Identify crystals in synovial fluids from written descriptions, including polarization characteristics. 10. Describe the process of obtaining bronchoalveolar lavage (BAL) samples, including safety precautions for analysis; state the purpose of BAL; and recognize types of cells that normally would be found in BAL specimens. Examination of all fluids should include observation of color and turbidity, determination of cell counts, and white blood cell (WBC) evaluation. Blood cell counts should be performed and cytocentrifuge slides should be prepared as quickly as possible after collection of the specimen, because WBCs begin to deteriorate within 30 minutes after collection.1 It is important to mix the specimen gently but thoroughly before every manipulation (i.e., counting cells, preparing any dilution, and preparing cytocentrifuge slides). Cell counts on fluids usually are performed using a hemacytometer (see Chapter 14); however, some automated instruments now are capable of performing blood cell counts on fluids. Automated cell counts are limited by the poor sensitivity of automated methods for specimens with low counts. Each instrument manufacturer should provide a statement of intended use that defines which body fluids have been approved by a regulatory agency for testing on the instrument.2,3 Care should be taken to observe the operating limits of these instruments and the volume limits for the fluid received. Red blood cell (RBC) counts on serous and synovial fluids have little clinical value4; relevant clinical information is obtained merely from the appearance of the fluid (grossly bloody, bloody, slightly bloody). The number of squares to be counted on the hemacytometer should be determined on the basis of the number of cells present. In general, all nine squares on both sides of the hemacytometer should be counted. If the number of cells is high, however, fewer squares may be counted.5 Each square equals 1 mm2. The formula for calculating the number of cells (see Chapter 14) is: Guidelines for counting are summarized in Table 17-1. TABLE 17-1 Guidelines for Counting Fluids RBC, Red blood cell; WBC, white blood cell. *Expected cell yield (WBC count for no. of cells recovered on slide): 0/mm3 for 0-70, 1-2/mm3 for 12-100, >3/mm3 for >100. The cytocentrifuge enhances the ability to identify the types of cells present in a fluid. This centrifuge spins at a low speed, which minimizes distortion of the cellular elements and provides a “button” of cells that are concentrated into a small area. The cytocentrifuge assembly consists of a cytofunnel, filter paper to absorb excess fluid, and a glass slide. These three components are fastened together in a clip assembly, a few drops of well-mixed specimen are dispensed into the cytofunnel, and the entire assembly is centrifuged slowly. The cells are deposited onto the slide, and excess fluid is absorbed into the filter paper, which produces a monolayer of cells in a small button (Figure 17-1). If a consistent amount of fluid is used when cytocentrifuge slides are prepared, a consistent yield of cells can be expected; this can be used to confirm the WBC or nucleated cell count. For example, if 5 drops of fluid (undiluted or diluted) is always used to prepare cytocentrifuge slides, a 100-cell differential count should be obtainable if the WBC or nucleated cell count is equal to or greater than 3/mm3. In all cases, the entire cell button should be scanned before the differential count is performed to ensure that significant clumps of cells are not overlooked. The area of the cell button that is used for performing the differential count is not important, but if the number of nucleated cells present is small, use of a “systematic meander” starting at one side of the button and working toward the other side is best. In case the number of cells recovered is small, the area around the cell button should be marked on the back of the slide with a wax pencil, or premarked slides should be used to prepare cytocentrifuge slides (see Figure 17-1). CSF is the only fluid that exists in quantities sufficient to sample in healthy individuals. CSF is present in volumes of 100 to 150 mL in adults, 60 to 100 mL in children, and 10 to 60 mL in newborns.6,7 This fluid bathes the brain and spinal column and serves as a cushion to protect the brain, as a circulating nutrient medium, as an excretory channel for nervous tissue metabolism, and as lubrication for the central nervous system. Normal CSF is nonviscous, clear, and colorless. A cloudy or hazy appearance may indicate the presence of WBCs (greater than 200/mm3), RBCs (greater than 400/mm3), or microorganisms.6,7 Bloody fluid may be caused by a traumatic tap, in which blood is acquired as the puncture is performed, or by a pathologic hemorrhage within the central nervous system. If more than one tube is received, the tubes can be observed for clearing from tube to tube. If the first tube contains blood but the remaining tubes are clear or progressively clearer, the blood is the result of a traumatic puncture. If all tubes are uniformly bloody, the probable cause is a subarachnoid hemorrhage. When a bloody sample is received, an aliquot should be centrifuged, and the color of the supernatant should be observed and reported. A clear, colorless supernatant indicates a traumatic tap, whereas a yellowish or pinkish yellow tinge may indicate a subarachnoid hemorrhage. This yellowish color sometimes is referred to as xanthochromia, but because not all xanthochromia is pathologic, the Clinical and Laboratory Standards Institute recommends avoiding the term and simply reporting the actual color of the supernatant (Figure 17-2 and Table 17-2).5 TABLE 17-2 Characteristics of Cerebrospinal Fluid
Body Fluids in the Hematology Laboratory
Performing Cell Counts on Body Fluids
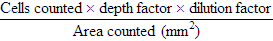
GROSS APPEARANCE
Test
Clear
Hazy
Blood-Tinged
Cloudy
Bloody
WBCs
0-200/mm3
>200/mm3
Unknown
High
Unknown
Dilution for counting cells
None
1 : 2 Türk
1 : 2 Türk
1 : 20 in Türk solution
1 : 2 in Türk solution
No. squares to count on hemacytometer
9
9
9
9 or 4
9 or 4
RBCs
0-400/mm3
Unknown
>400/mm3
Unknown
>6000/mm3
Dilution for counting cells
None
None
None
None
1 : 200 in isotonic saline
No. squares to count on hemacytometer
9 large
9 large
9 or 4 large
4 large or 5 small
5 small
Cytospin dilution (0.25 mL [5 drops] of fluid)*
Undiluted
Dilute with saline to 100-200/mm3 nucleated cell count
Straight or by nucleated cell count
Dilute with saline to 100-200/mm3 nucleated cell count
Dilute by nucleated cell count; if RBC count >1 million/mm3, make a push smear and differentiate cells that are pushed out on the end
Preparing Cytocentrifuge Slides
Cerebrospinal Fluid
Gross Examination
Traumatic Tap
Pathologic Hemorrhage
Clear supernatant
Colored or hemolyzed supernatant
Clearing from tube to tube
Same appearance in all tubes
Bone marrow contamination
Erythrophages
Cartilage cells
Siderophages (may have bilirubin crystals)
Body Fluids in the Hematology Laboratory