▪ 24A Trimodality Therapy: Selective Bladder Preservation in the Treatment of Muscle-Invasive Bladder Cancer
Mohamed E. Abazeed
Jason A. Efstathiou
Niall M. Heney
Donald S. Kaufman
William U. Shipley
EXTERNAL BEAM RADIATION THERAPY ALONE
Beginning in the 1960s, the most common method of bladder-sparing treatment was megavoltage external beam radiation therapy. In the United States, radiation treatment was generally reserved for patients judged unfit for cystectomy on the basis of age, comorbid conditions, or disease extent. These negative-selection criteria likely contributed to the poor results achieved with radiation therapy alone (
Table 24A.1). In addition, approximately 15% of the patients are excluded from treatment by radical surgery because at the time of operation, previously unrecognized extravesical tumor is found. Thus, in cystectomy series, unlike the radiation series, some patients with advanced local spread of tumor are excluded. Despite this, radiation alone at doses of 45 to 60 Gy showed pathological complete response (CR) rates and local control rates of ˜40% in the early series (
5).
By 1985, of four randomized trials that compared cystectomy (and preoperative radiation therapy) with external beam treatment alone, only one reported a statistically significant survival advantage for immediate cystectomy (
6). In a prospective multicenter trial at the Institute of Urology in London, patients were randomized to undergo either 40 Gy of radiation followed by total cystectomy or 60 Gy of radiation followed by salvage cystectomy for persistent or recurrent tumor (
7). This trial showed no difference in the 5- and 10-year survival rates for the 98 patients randomized to immediate cystectomy (39% and 19%, respectively), compared to the 91 patients randomized to radiation with salvage surgery, with the comparable figures 28% and 15%. In 1991, the Danish National Bladder Cancer Group reported no statistical survival difference in overall survival in two arms of a similar study that involved 183 patients (
8). The pelvic failure rate was, however, significantly lower in the group treated by immediate cystectomy compared to those treated by radiation therapy. The incidence of metastatic disease was similar in both groups, 32% and 34% at 5 years.
The National Bladder Cancer Group reported a randomized trial of 72 patients in which there was no difference in the 5-year survival rate or the rate of distant metastasis for patients randomized to immediate cystectomy (27% and 38%, respectively) compared with those undergoing primary radiation therapy with cystectomy only for recurrence (40% and 31%, respectively). The median follow-up of that phase III study was 66 months. Thus, in the two trials that reported the incidence of the subsequent development of distant metastases, there was no increased rate among those patients receiving radiation with deferred cystectomy for salvage, and in only one of the four randomized trials comparing immediate cystectomy to radical radiation therapy with cystectomy deferred for salvage was there a statistically significant survival advantage (observed in the smallest trial that included 67 patients with advanced stage T3 tumors) (
6).
The efficacy of accelerated (twice-a-day) radiation regimens without concomitant chemotherapy in eradicating a primary bladder tumor was evaluated in a randomized phase III trial at the Royal Marsden Hospital with radiation as monotherapy (
9). The accelerated fractionation schedule did not improve on the efficacy of conventional fractionation for patients with T2 and T3 bladder cancer (the 5-year overall survival was 37% for accelerated fractionation and 40% for conventional fractionation) and accelerated fractionation was associated
with increased acute bowel reactions (RTOG grade 2 or 3 bowel toxicity was noted in 44% of accelerated fractionation patients compared to 26% of conventional fractionation patients [
p = 0.001]). Finally, radiation treatment schedules that use a relatively large dose per fraction (more than 2 Gy) have been shown to produce a late toxicity rate in the bladder and intestine of 20% to 25% (
10). This is distinctly less satisfactory than the excellent bladder tolerance achieved with conventional radiation.
Modern treatment techniques involve the delivery of radiation to the whole small pelvis, with the external and internal iliac lymph nodes in the target volume, for a total dose of 40 to 45 Gy in 1.8- to 2.0- Gy fractions over 4 to 5 weeks. Subsequently, the target volume is reduced to deliver a final boost of 20 to 25 Gy in 10 to 15 fractions to the primary tumor. Some protocols call for partial bladder radiation as the boost volume, if the location of the tumor in the bladder can be satisfactorily determined by use of cystoscopic mapping, selective mucosal biopsies, and imaging information from computed tomography (CT) or magnetic resonance imaging (MRI). When twice-a-day radiation protocols are to be used, a dose per fraction of 1.5 to 1.6 Gy has been shown to be quite well tolerated when combined with concurrent cisplatin-containing chemotherapy (
11,
12). With concurrent cisplatin-containing chemotherapy, total whole bladder doses from 50 to 55 Gy and total doses to the bladder tumor volume of 65 Gy have been widely used. In the United Kingdom, daily doses of 2.5 to 2.75 Gy per fraction over 4 weeks to a total dose of 50 to 55 Gy have been the standard. However, available information suggests that the higher dose per fraction leads to a higher rate of late bladder complications.
The bladder is not a fixed organ, with significant interday variability in location and volume. This results in a number of logistic problems which require the careful attention of the radiation oncologist to ensure adequate coverage of the bladder tumor. Recent studies have identified substantial movement of the bladder during the course of external beam radiation
therapy, and as a result of these findings a minimum margin of 2 cm to 2.5 cm around the target volume to the edge of the light field is necessary (
13,
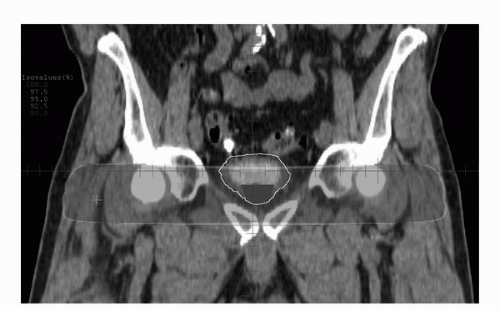
14). When three-dimensional (3D) conformal external beam radiation therapy is to be used, treating the patient with an empty bladder minimizes bladder motion (
Fig. 24A.2). The clinical target volume expansion to the planning target volume should be 1.5 to 2.0 cm and possibly even greater superiorly (
13). Total bladder tumor doses of higher than 65 Gy seem appropriate. However, because of the increased problems with bladder organ motion and with the fact that the majority of patients are now treated with concurrent cisplatin-containing chemotherapy, escalation in total dose above 65 Gy should be done only in the investigational setting.
New approaches are being explored to improve the conformality and precision of radiation delivery to the bladder including interstitial radiation therapy (brachytherapy), partial bladder radiation, and the use of on-board image guidance. Brachytherapy, with implant doses of 40 Gy coupled with external beam radiation doses of 30 Gy or more, allows for the delivery of a higher biologic dose of radiation to a limited area of the bladder within a short period. The majority of reported series include patients who first underwent maximum tumor debulking either by partial cystectomy or transurethral resection. This approach is reserved for patients with solitary bladder tumors <5 cm in diameter; 5-year survival rates of 62% to 84% have been reported, with disease-specific survival approaching 80% (
10,
15,
16). These results suggest that in carefully selected patients, brachytherapy produces high local control rates and can result in excellent survival.
Another approach to optimizing radiation treatment is partial bladder radiation, which is currently being investigated in BC2001. This is a phase III trial utilizing a 2 × 2 design with patients randomized to one or both of two randomizations: (a) radiation alone or radiation with 5-fluorouracil (5-FU) and mitomycin C (MMC) and (b) radiation to the tumor and whole bladder or partial bladder (15% dose reduction to uninvolved bladder). Preliminary results indicate no measurable differences in toxicity or recurrence rates at 24-month follow-up. Interestingly, the extent of bladder volume reduction after radiation therapy appeared to be less in the partial bladder group. However, these results need to be confirmed and extended, as it remains unclear whether such results will lead to clinically measurable differences in outcomes.
Additional improvements are likely to be derived from treatment precision. For example, the technology of online mega-voltage cone-beam computed tomography (MV-CBCT) imaging is currently used in many institutions to generate a 3D anatomical dataset of a patient in treatment position. It utilizes an accelerator therapy beam captured by an electronic portal imager to account for organ motion and setup variations. CBCT bladder volumes significantly differ from planning CT counterparts suggesting that the significant variations in bladder and planned target volume and position can be minimized using image-guided radiation treatment (
17). The clinical significance of these findings remains to be elucidated.
TRIMODALITY TREATMENT WITH SELECTIVE BLADDER SPARING
Local control rates with radiation alone for muscle-invading tumors were disappointingly low, and radiation as monotherapy has largely been abandoned (
5,
18,
19,
20). Monotherapies of various types are in general unsuccessful in curing patients of invasive bladder cancer. For example, Barnes et al. (
21) reported a 27% 5-year survival in 85 patients with welldifferentiated and moderately differentiated T2 TCCs treated with transurethral resection alone. Sweeney et al. (
22) found that only 19% of patients with muscle-invasive tumors were selected for treatment by partial cystectomy, and these had a local recurrence rate of 38% to 78%. Hall and Roberts (
23) reported durable complete remissions in only 17% of patients utilizing chemotherapy alone. The local control rates for the monotherapies noted above are inadequate when compared to RC with its local control rate of ˜90%.
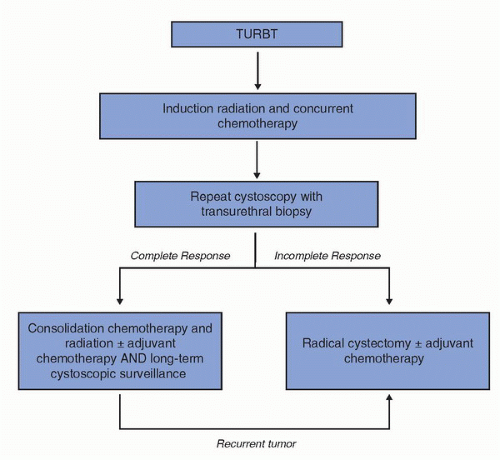
The low early control rates with radiation therapy and other forms of monotherapy led several groups to assess the value of combining all three modalities, TURBT, radiotherapy, and chemotherapy. Attempts at bladder sparing are patient selective. Induction chemoradiation must achieve a pathological CR, as measured by follow-up cytology and cystoscopic biopsies. Individuals who have a CR receive consolidation chemoradiation (total dose of 64-65 Gy) and patients who have negative bladder biopsies following consolidation chemoradiation are eligible for adjuvant chemotherapy. If residual disease is found at any phase of the algorithm, cystectomy is recommended as an immediate step in treatment, followed usually by adjuvant chemotherapy.
Over the last 25 years, single institutions in North America and Europe and multi-institutional cooperative groups including the Radiation Therapy Oncology Group (RTOG) and the Southwestern Oncology Group (SWOG) have enrolled >1,000 patients in bladder-preservation protocols. Several variables have been tested including radiation-sensitizing chemotherapeutic agents, alternative radiation therapy fractionation schemes (see above), and neoadjuvant and adjuvant chemotherapies.
The combination of transurethral resection, radiation, and chemotherapy has yielded better results than any of the monotherapies, with an improved clinical CR rate (
Tables 24A.2 and
24A.3). Moreover, substantial improvements in local control and distant metastasis rates have been achieved with combined modality therapy (
Table 24A.4). This generally consists of TURBT for debulking, with the goal a visibly complete tumor removal, followed by radiation treatment with concurrent radiosensitizing chemotherapy. In the studies reported, the most commonly used radiosensitizing drugs were cisplatin, 5-FU, and paclitaxel, used either singly or in various combinations.
Several lines of evidence indicate that newer drugs have considerable activity against metastatic bladder cancer (
Table 24A.5). The list includes cisplatin, methotrexate, vinblastine, ifosfamide, doxorubicin, gemcitabine, and paclitaxel as the principal agents (see
Chapter 26). Not surprisingly, combinations of these drugs have been shown to be more effective than single agents in the percentage of CRs achieved. With the use of combination chemotherapy in advanced measurable disease, CR has become a common achievement as compared to a 15% to 20% rate of partial remission utilizing a variety of single drugs, and with complete remissions only rarely observed with single-agent treatments.
Successful approaches have evolved over the last two decades following the initial reports of the effectiveness and safety of cisplatin against TCC and reports of added efficacy when it is given concurrently with radiation. From 1981 to 1986, the National Bladder Cancer Group first used cisplatin as a radiation sensitizer in 68 patients with muscularis propria-invading bladder cancer who were unsuitable for cystectomy. In a multicenter protocol, this approach was shown to be feasible and safe (
24). Furthermore, the long-term survival rate with stage T2 tumors (64%) and even for stage T3-T4 tumors (22%) was encouraging. This early result with concurrent cisplatin and pelvic irradiation was validated by the NCI-Canada randomized trial of radiation (either definitive or pre-cystectomy) with or without concurrent cisplatin for patients with T3 bladder cancer. The Canadian study showed a significant improvement in pelvic tumor control (67% vs. 47%) in the patients who were assigned cisplatin (
25). Moreover, single-institution studies showed that the combination of a visibly complete TURBT followed by radiation therapy or radiation therapy concurrent with chemotherapy led to improved local control (
26,
27). These findings led the RTOG to develop the algorithm for bladder preservation of an initial TURBT of as much of the bladder tumor as is safely possible followed by a combination of radiation with concurrent radiosensitizing chemotherapy. One novel aspect of such a program is the selection of patients for bladder preservation on the basis of the initial response of each individual patient’s tumor to concurrent chemoradiotherapy. Thus, bladder conservation was reserved for those patients who had a clinical CR to concurrent chemotherapy and radiation. Prompt cystectomy was recommended for those patients whose tumors responded only incompletely or who subsequently developed an invasive tumor (
Fig. 24A.1). All of the protocols developed at the Massachusetts General Hospital (MGH) or within RTOG since 1986 call for RC at the first sign of failure of local control. Up to one third of the patients entering a potential bladder-preserving protocol with trimodality therapy (initial TURBT followed by concurrent chemotherapy and radiation) will require RC (
28).
Eligibility criteria for bladder sparing include (a) histological proof of invasion of the muscularis propria, (b) adequate renal function, (c) the absence of tumor-associated hydronephrosis, (d) medical fitness for cystectomy, if necessary, (e) absence of extensive carcinoma in situ associated with the invasive tumor, and (f) the absence of malignant lymphadenopathy on imaging studies utilizing CT or MRI, with biopsy as necessary. Patients must adhere to a strict regimen of surveillance cystoscopy after treatment. Key exclusion criteria include renal insufficiency, which precludes the use of platinum-based chemotherapy, and the presence of tumor-associated hydronephrosis, which appears to predict a poor response to induction chemoradiotherapy in preliminary RTOG studies.
Protocols carried out between 1986 and 2009 at the Massachusetts General Hospital included as complete a TURBT as possible, followed by radiation and concomitant chemotherapy (
29,
30). In an early protocol, we utilized neoadjuvant multidrug systemic chemotherapy in an effort to reduce the appearance of distant metastases. However, the 5-year and 10-year metastasis-free survival rates were not influenced by the addition of two cycles of neoadjuvant methotrexate, cisplatin, and vinblastine (MCV) chemotherapy. We have not used neoadjuvant chemotherapy in subsequent protocols, directing our attention instead to adjuvant chemotherapy.
Phase II and III protocols with concurrent chemoradiotherapy are listed in
Tables 24A.2 and
24A.3 and are described, in part, here. The first RTOG study, RTOG 8512, included 42 patients treated with once-daily radiation treatment and concurrent cisplatin, yielding a 5-year survival of 52% (
31). This treatment was well tolerated and resulted in 42% of the patients achieving long-term survival with an intact bladder. RTOG studies 8802 and 8903 utilized MCV chemotherapy as neoadjuvant treatment. In the latter study, patients were treated on a phase III trial with or without two cycles of MCV prior to the combination of cisplatin and once-a-day radiation (
32). There was no improvement in survival or in local tumor eradication as a result of neoadjuvant therapy (
33). With a median follow-up of 5 years the overall survival was 48% in patients treated with MCV and 49% in patients who received no neoadjuvant treatment. The cystoscopic CR rate was 61% in the MCV arm and 55% in the control arm, not statistically significant. At 5 years, metastases were present in 35% of the patients who received MCV and 42% of the patients who received no neoadjuvant chemotherapy. This difference was not statistically significant. The toxicity of the MCV arm was considerable, with only 67% of patients able to complete the planned treatment. This phase III study was not sufficiently powered to settle the question of the place of neo-adjuvant chemotherapy in patients undergoing bladder-preserving therapy, but neither RTOG nor MGH has revisited the question of the effectiveness of neo-adjuvant chemotherapy.
Houssett et al. from the University of Paris reported on 120 patients with stage T2-T4a bladder cancer. The treatment consisted of TURBT followed by cisplatin and 5-FU given concurrently with twice-a-day hypofractionated radiation. They reported a 63% overall survival rate (
34).
Investigators at the University of Erlangen have the largest bladder-sparing series to date, 415 patients treated from 1982 to 2000 (
35). This report included 126 patients who received radiation without any chemotherapy and 89 patients who were not clinical stage T2 to T4 but classified as “high-risk T1.” The CR rate of all 415 patients was 72% and local control of the bladder tumor after the CR without a muscle invasive relapse was maintained in 64% of the patients at 10 years. The 10-year disease-specific survival was 42% and more than 80% of these survivors preserved their bladder. This series, not randomized, included the use of radiation with no chemotherapy (126 patients), radiation with concurrent cisplatin (145 patients), radiation with concurrent carboplatin (95 patients), and radiation with concurrent cisplatin plus 5-FU (49 patients). The CR rates in these four treatment protocols were 51%, 81%, 64%, and 87%, respectively (
36,
37). The 5-year actuarial survival with an intact bladder in these four
protocols were 38%, 47%, 41%, and 54%, respectively. These results suggest strongly that chemoradiotherapy when given concurrently is superior to radiation therapy alone, that carboplatin is less radiosensitizing than cisplatin, and that cisplatin plus 5-FU may be superior to cisplatin alone. The authors recognized that their study was compromised by the absence of randomized trial data.
In RTOG 9906, paclitaxel was added to the induction and consolidation chemoradiation regimens, based on encouraging results with paclitaxel in advanced bladder cancer, and its putative radiation-sensitizing effect. This study also used cisplatin and gemcitabine in the adjuvant setting; cisplatin and gemcitabine had been previously shown to have equal efficacy and less toxicity compared to MVAC. The postinduction CR rate was an impressive 81% and the 5-year actuarial overall and disease-specific survival rate was 56% and 71%, respectively. The addition of paclitaxel to the regimen resulted in a greater percentage of grade 3-4 toxicity, mostly in the form of diarrhea (15%) during induction.
From 1994 to 1998, twice-daily radiation therapy was introduced into RTOG protocols with concurrent cisplatin or with cisplatin plus 5-FU as radiosensitizers (
38). From 1999 to 2002, twice-a-day radiation concurrent with cisplatin and paclitaxel as radiosensitizers along with adjuvant cisplatin and gemcitabine was evaluated. The latest North American protocol for bladder-sparing treatment (RTOG 0233) has recently met its accrual goal. This is a randomized phase II study comparing two combinations of radiosensitizing chemotherapy (cisplatin plus paclitaxel vs. cisplatin plus 5-FU), each given concurrently with an induction course of twice-daily radiation treatment (http://rtog.org/members/protocols/0233/0233.pdf). This is followed in patients whose tumors initially responded completely by consolidation chemoradiation and in those with incompletely responding tumors by RC. All patients are then to undergo a three-drug adjuvant treatment consisting of cisplatin, gemcitabine, and paclitaxel (
38). The results of RTOG 0233 are pending.
Gemcitabine has been tested in bladder-treatment protocols, with promising results. In a phase I trial from the University of Michigan, 23 mostly T2 patients were treated with gemcitabine and concurrent daily radiation. At a median follow-up of 5.6 years, an impressive 91% CR rate was observed, and the 5-year actuarial estimates of survival include a bladder-intact survival of 62%, an overall survival of 76%, and a diseasespecific survival of 82% (
39). Locoregional failure occurred in seven patients (30%), which were successfully salvaged by RC (five patients) or intravesical therapy (
n = 2). RTOG 0712 is a randomized phase II trial that will compare cisplatin and 5-FU and the RTOG approach of twice daily fractionation to gemcitabine with concurrent daily radiation (the Michigan approach) (http://rtog.org/members/protocols/0712/0712.pdf). MMC has also shown some activity in the combined-modality setting. In a British phase II trial, MMC and 5-FU with concurrent radiation was delivered to 41 patients with invasive bladder cancer, resulting in a CR rate of 59%. At a median follow-up of 50.7 months, 39% of the patients were alive and 88% of these patients maintained a functioning bladder, notwithstanding the enrollment of patients with poor prognostic factors (20/41 patients were with hydronephrosis) (
40). The BC2001 protocol is also testing the efficacy of MMC and 5-FU. BC2001 is a phase III trial utilizing a 2 × 2 design with patients randomized to one or both of two randomizations: (a) radiation alone or radiation with 5-FU and MMC and (b) radiation to the tumor and whole bladder or partial bladder (15% dose reduction to uninvolved bladder). Preliminary results indicate no measurable differences in toxicity at 24-month follow-up.
The MGH experience with 348 patients with invasive bladder cancers with clinical stages T2-T4a entered on successive prospective protocols has recently been updated (
29). A common feature of all the protocols was early bladder tumor response evaluation and the selection of patients for bladder conservation on the basis of their initial response to TURBT combined with chemotherapy and radiation. Bladder conservation was reserved for those who had a complete clinical response at the midpoint in therapy (after a radiation dosage of 40 Gy). Approximately two thirds of the total then received consolidation with additional chemotherapy and radiation to a total tumor dose of 64 to 65 Gy. Incomplete responders were advised to undergo RC, as were patients whose invasive tumors persisted or recurred after treatment. In these phase II and phase III protocols, the scheduling of the chemoradiation varied. In the one phase III study, all patients underwent TURBT with patients then randomized to neoadjuvant or no neoadjuvant chemotherapy. All patients were treated with concurrent chemotherapy (which always included cisplatin) and radiation therapy.
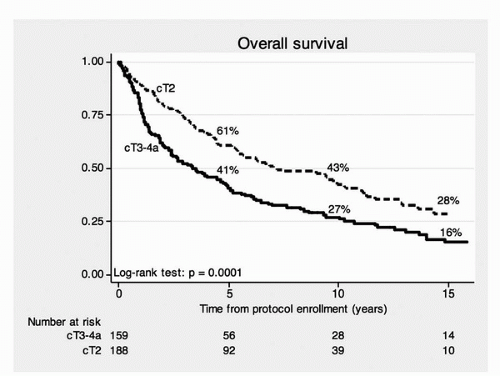
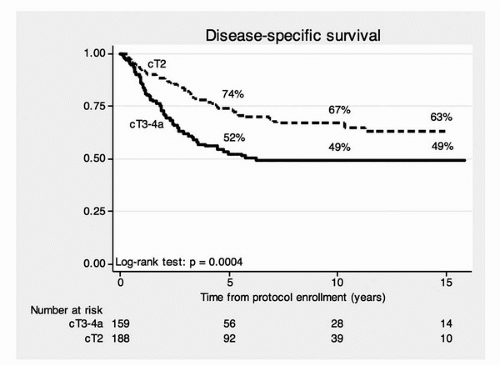
The median follow-up for all surviving patients was 7.7 years. The 5-, 10-, and 15-year actuarial overall survival rates were 52%, 35%, and 22%, respectively (stage T2, 61%, 43%, and 28%; stage T3-T4a, 41%, 27%, and 16%). The 5-, 10-, and 15-year disease-specific survivals were 64%, 59%, and 57% (stage T2, 74%, 67%, and 63%; stage T3-T4a, 53%, 49%, and 49%). The overall and disease-specific survival rates stratified by clinical stage are shown in
Figures 24A.3 and
24A.4. The clinical stage (
Figs. 24A.3 and
24A.4) was
significantly associated with overall survival (
p = 0.0001) and disease-specific survival (
p = 0.0004). The use of neoadjuvant chemotherapy, utilizing MCV, did not significantly affect overall survival, disease-specific survival, or distant metastasis-free survival. The 5-, 10-, and 15-year disease-specific survival rate for the 102 patients undergoing cystectomy were 55%, 44%, and 44%, respectively. This indicates the very important contribution of prompt salvage cystectomy for disease control in the 102 patients who required salvage cystectomy. The 5-, 10-, and 15-year disease-specific survivals with an intact bladder were 60%, 45%, and 36% (T2, 69%, 52%, and 46%; T3-T4a, 48%, 36%, and 26%). No patient required cystectomy due to bladder morbidity. The actuarial 5-, 10-, and 15-year overall survival and disease-specific survival for all 348 patients and for some clinically important subgroups are shown in
Table 24A.6. The current schema for trimodality treatment of muscle-invading bladder cancer is provided in
Figure 24A.1.
The value of complete TURBT in bladder-sparing therapy is demonstrated in
Table 24A.7. Of the 348 patients followed, 227 underwent a complete TURBT and 116 had an incomplete TURBT. Patients who underwent a complete TURBT had improved CR (79% vs. 57%,
p < 0.001), overall survival (57% vs. 43%,
p = 0.003), disease-specific survival (68% vs. 56%,
p = 0.03), and lower rates of cystectomy (22% vs. 42%,
p <0.001) compared to those with an incomplete TURBT. These data demonstrate an association between complete TURBT and improved outcomes in bladder-sparing therapy.
Comparing our results to those of contemporary RC series is confounded by the discordance between clinical TURBT staging and pathological (cystectomy) staging. A prospective evaluation from Stockholm has documented that clinical staging is more likely to underestimate the extent of the disease with regard to penetration into muscularis propria or beyond that is pathological staging (
41). Thus, if any favorable outcome bias exists, it is in favor of the pathologically reported RC series. The University of Southern California recently reported on 633 patients undergoing RC with pathologic stages T2 to T4a with an actuarial overall survival rate at 5 years of 48% and 10 years of 32% (
4). The Memorial Sloan-Kettering Cancer Center contemporary RC series showed that in 184 patients with tumors of pathologic stage P2-P4, the 5-year overall survival rate was 36%. The actuarial 5-year survival rate of all 269 patients undergoing RC with pathologic stages ranging from P0 to P4 in this series was 45% (
42). The results of these and other more contemporary cystectomy series for muscle-invading bladder cancer are similar to the MGH series as well as those from the University of Erlangen (
35) and the RTOG (
33) (
Table 24A.2). This similarity in survival is likely in part due to the prompt use of salvage cystectomy when necessary in the selective bladder-preservation series. The disease-specific survival rate of 44% at 15 years in the MGH series for patients having cystectomy underscores the need for close urologic follow-up with cystoscopic surveillance and prompt bladder removal when clinically indicated.