INTRODUCTION
Expectant optimism is now pervading the field of urothelial cancer as we anticipate that we will soon be soaring above the plateaus established with cisplatin-based chemotherapy in the 1980s and finally have new agents approved for the treatment of urothelial carcinoma. Immune checkpoint inhibitors, which are transforming the field of oncology, are showing evidence of clinical activity in early clinical trials in this disease (1). In addition, there are several ongoing trials of targeted agents including fibroblast growth factor (FGF) receptor inhibitors and vascular endothelial growth factor (VEGF) inhibitors currently in clinical trials with the goal of obtaining US Food and Drug Administration (FDA) approval. Our fundamental understanding of the biology of urothelial cancer is changing as well, with molecular characterization suggesting that urothelial cancer is no longer only one disease (2). These nascent technologies suggest we will soon be able to personalize therapy for urothelial cancer and reliably predict which patients will benefit from specific chemotherapy and/or other targeted agents, transforming our current treatment of urothelial cancer.
OVERVIEW
The urinary tract conveys urine from the confluence of urinary tubules in the renal papillae to the outside world. This entire path is lined by a specialized epithelial surface known as the urothelium, which is composed primarily of transitional cells, and extends from the renal pelvis through the ureters, bladder, and urethra. In males, it also lines the terminal prostatic ducts and prostatic urethra. Although tumors arising from the urothelium can involve any organ along this path, about 90% of these cancers arise in the urinary bladder.
EPIDEMIOLOGY
Urothelial cancer is the fifth most common cancer diagnosis in the United States and is strikingly related to cigarette smoking. In 2015, about 80,000 new cases were expected, with about 74,000 arising in the bladder. Altogether, these cases account for just over 18,000 deaths (3). These incidence figures are somewhat misleading, however, because it is a historical anomaly that only in the bladder are histologically bland hyperplastic lesions counted as cancers. In other sites, such lesions would be counted as benign or at most premalignant, and thus the incidence figures include many patients with lesions that do not meet the conventional definition of malignancy. Imagine what the incidence figures for colon cancer would be if every patient with a polyp was counted as a case of colon cancer! Many such lesions recur; however, few progress to true malignancy. Thus, it is critically important to separate risk models that are designed to predict recurrence from those that predict progression, which is far more biologically significant. Because of this anomaly of classification, the literature on “risk of bladder cancer,” both for incidence and recurrence, must be interpreted very carefully.
In contrast to most other carcinomas, the majority of patients with urothelial cancer (even after excluding the low-grade papillary “cancers”) have early-stage, potentially curable disease at presentation. Only about 20% of patients present with disease that invades into the muscle wall. Fewer than 5% of patients present with locally advanced (ie, clinically extravesical) disease, and another 5% or so present with clinically apparent metastatic disease. Once clinically metastatic, urothelial cancer is remarkably aggressive, exhibiting a natural history reminiscent of small cell carcinoma of the lungs: untreated, the survival is measured in weeks; it is markedly chemotherapy sensitive; responses are typically short lived; the brain is a typical “sanctuary” site of relapse after response to initial therapy; and cure of patients with distant metastases, although well documented, remains anecdotal.
The ready accessibility of urine and the fact that the urothelium itself can be accessed via minimally invasive cystoscopy makes urothelial cancer an important context for understanding the processes of carcinogenesis and clonal evolution and an important platform for the development of human gene therapy.
The current state of the art is rather sobering:
Careful patient selection has made it possible for some patients to have organ preservation, but this strategy clearly results in some patients dying unnecessarily.
Although the combination of chemotherapy and surgery does improve the cure fraction for patients with locally advanced disease, a disturbing fraction (30%-40% even at referral centers) of patients with invasive disease but no detectable involvement beyond the bladder at the time of diagnosis still succumb to the disease.
There have been no substantive advances in systemic treatment since the introduction of methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) in the early 1980s, and indeed, reported outcomes for patients with metastatic urothelial cancer are getting worse as marginally effective systemic therapies (such as gemcitabine and carboplatin) are now widely used.
However, there is optimism for the following reasons:
Recent developments in immunology with checkpoint inhibitors suggest their utility in urothelial cancer.
The molecular characterization of urothelial cancer may help us choose which patients are most likely to benefit from a specific therapy, leading to a personalized approach to care.
CLASSICAL EPIDEMIOLOGY
Malignant transformation of the urothelium parallels observations with other epithelial tumors (4), in that it is distinctly uncommon before age 40 and has a peak incidence in the seventh decade. The male-to-female ratio is about 3:1, reflecting, in part, differences in exposure to smoking and industrial toxins. Malignant transformation is the result of a multistep process in which multiple genes are implicated. The appearance of clinical cancer typically follows carcinogenic exposure by decades. Fluid intake is inversely associated with risk (5), supporting the concept that contact time of excreted carcinogens with the urothelial surface contributes to carcinogenesis.
There is a striking correlation of urothelial cancer incidence with exposure to certain environmental toxins. About half of all cases are related to smoking. Industrial exposures, especially to petrochemicals, account for another 10% to 15%. Many occupations with “chemical” exposures have been linked to an excess risk of urothelial cancer. An association with aniline dye exposure was noted more than a century ago, and many aromatic amines (prototypically β-naphthyl amine) have now been shown to be potent urothelial carcinogens. Cigarette smoke is rich in both aromatic amines and the highly reactive bladder toxin acrolein. Another source of acrolein, which is strongly linked to urothelial carcinogenesis, is the metabolism of cyclophosphamide and ifosfamide. In one report, prolonged exposure to oral cyclophosphamide (as was a common intervention for some forms of cancer and for autoimmune disease in the 1970s) increased the risk of bladder cancer by a factor of nine (6). Also noteworthy are exposures to the analgesic phenacetin and to the plant toxin–related “Balkan nephropathy” in which the risk of upper tract tumors is increased (7,8).
As is generally true for epithelial carcinogenesis, chronic irritation and inflammation have also been associated with malignant transformation. This is seen prototypically in settings such as staghorn calculus and other cases of chronic urolithiasis, chronic bladder catheterization (a particularly distressing complication of spinal cord injury (9) or congenital malformations), and classically, chronic schistosomiasis in the Middle East. Urothelial cancers that arise in the context of chronic irritation typically show squamous differentiation.
Assessment of genomic variations, as well as functional assessment of certain metabolic pathways, is now routinely integrated with classical aspects of cancer risk assessment. As might have been anticipated from the classical epidemiologic studies of environmental exposures, it has now been confirmed that polymorphisms of various genes involved in xenobiotic metabolism are correlated with the risk of developing bladder cancer. However, these pathways are complex, and the impact of genetic changes is of course context dependent. A classic example of this is provided by N-acetyltransferase 2 (NAT-2)–mediated N-acetylation, which constitutes a detoxification step for some carcinogens but can be an activation step for others (10,11). A meta-analysis suggests a possible role for some of these variants in risk of bladder cancer and clearly demonstrates an interaction with smoking status (12), as would be expected on the hypothesis that these genes are important for the response to smoking-induced DNA damage (13).
Telomere length, which also contributes to the maintenance of genetic integrity, may also be relevant to urothelial carcinogenesis because patients with bladder cancer have been found to have shortened telomeres or telomerase abnormalities (14,15).
TUMOR BIOLOGY
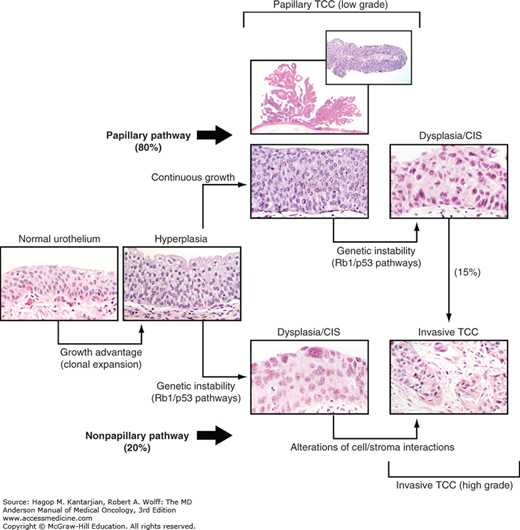
A dual track concept has been proposed in urothelial cancer carcinogenesis (Fig. 36-1) (16). The majority of urothelial cancers, approximately 80% to 85%, arise from a hyperplastic epithelium and most commonly present as low-grade papillary tumors. Although these tumors tend to recur, they are much less likely to evolve into a more aggressive form of urothelial cancer. Earlier studies suggested that preservation of E-cadherin expression is classic in these tumors and associated with a low risk of recurrence (17), with additional studies suggesting that ratios of E-cadherin to matrix metalloproteinase-9, which is a surrogate for epithelial to mesenchymal transformation (EMT), are inversely correlated with outcome (18,19). Additional proliferative drivers including FGF receptor (FGFR)-3 have been observed in a much higher frequency in these superficial papillary urothelial cancers of low malignant potential (20).
Because the bladder is readily accessible, with most patients presenting before life-threatening progression is apparent, bladder cancer is well suited for studying the details of the molecular pathogenesis of transformation, invasion, and metastasis. Molecular genetics investigations have revealed that numerous loci are lost and have confirmed that clonal expansion is an early event; that is, morphologically normal urothelium in bladders harboring urothelial tumors is genetically altered, and multifocal disease generally represents multifocal expansion of a preexisting clonal lesion (21). Thus, the classical notion of “field carcinogenesis” is amply confirmed by these molecular genetic studies. These studies demonstrate that early carcinogenesis involves relatively few key loci that provide the fertile context for more generalized chromosomal instability that is apparent in grossly established invasive tumors.
Following along the dual track concept of bladder carcinogenesis, the nonpapillary pathway is associated with early genetic instability (see Fig. 36-1). These flat, infiltrative tumors typically behave more aggressively and invade the muscularis propria with a much higher probability of impacting survival. Loss of the retinoblastoma gene (RB) or any inactivation of RB function is clearly associated with an adverse prognosis in these tumors (22). The p53 gene is also frequently mutated in these tumors (23) and appears to interact with RB (24). Abnormal p53 immunohistochemical staining is highly correlated with a nonfunctional mutation associated adverse prognosis (25).
Tumors from the nonpapillary pathway typically have mesenchymal features associated with their invasive nature. However, recent studies suggest that the EMT concept is indeed more complex as some epithelial markers, including TP63 expression, have been associated with adverse outcome in the setting of invasive disease (26,27). TP63 expression has been noted to be at high levels within the basal layer of the urothelium, which typically contains the stem cell compartments. In addition, most p63 isoforms are felt to act as dominant negatives, potentially suppressing p53-dependent transactivation (28). Recent studies using gene expression profiling continue to suggest a strong role for p63 controlling basal gene expression in these lethal basal tumors (2).
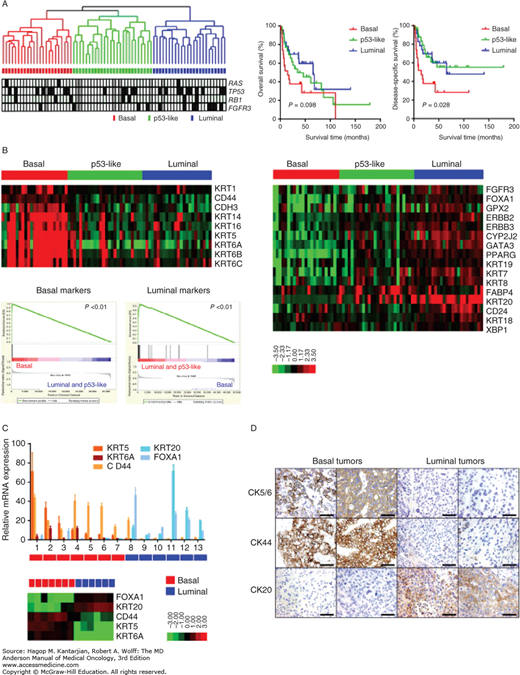
Recently, three distinct subtypes of urothelial cancer have been identified using gene expression profiling (GEP) (Fig. 36-2) (2,29). Basal urothelial carcinomas, which have immunohistochemical markers associated with the basal layer, are often characterized by TP63 activation and rapid proliferation with more aggressive disease and may be prognostic for poor outcomes. The angiogenic signature is expressed in the basal subtype. Luminal urothelial carcinomas, which share immunohistochemical markers associated with the umbrella cell layer, show evidence of PPARγ activation. Enrichment of FGFR3 mutations has been noted in this group of tumors. A third group of p53-like urothelial cancers show a high degree of resistance to typical chemotherapy agents and are associated with a stromal infiltrate. A recent series of patient treated on clinical trial suggests a high incidence of bone metastasis in this subtype (in press, Evr. Urol.). Furthermore, data from this clinical trial suggest that clinical outcomes are improved when the basal subtype tumors are treated with neoadjuvant chemotherapy, suggesting this is a potential predictive marker for clinical outcomes. Therefore, subtyping strategies may aid in the development of personalized treatment for urothelial cancer and appropriate selection of patients for systemic chemotherapy and specific targeting agents.
FIGURE 36-2
Molecular characterization of bladder cancer suggesting three intrinsic subtypes: basal, luminal, and p53-like. (Reproduced with permission from Choi W, Porten S, Kim S, et al: Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy, Cancer Cell 2014 Feb 10;25(2):152-165.)
The use of immune checkpoint inhibitors appears to be transforming the world of cancer, including the field of urothelial cancer. Early results from clinical trials suggest that response rates with programmed cell death (PD)-1 inhibitors maybe as high as 20% to 40% (1). Combined checkpoint inhibition using PD1 inhibitors in combination with cytotoxic T lymphocyte-associated antigen 4 (CTLA4) antibodies potentially may yield improved responses, although trials are still accruing in this group of patients. Expression of PD ligand 1 (PDL1) either on the tumor cell or on the lymphocyte infiltrate has been proposed as one method of determining which patients are most likely respond to checkpoint inhibition. Because the response rates in PDL1 negative disease maybe anywhere from 15% to 20% and PDL1 expression is dynamic and may be influenced by chemotherapy and intravesical agents including bacillus Calmette-Guérin (BCG), the clinical utility of this biomarker remains problematic. Approval of a checkpoint inhibitor in the treatment of urothelial cancer is an event that is highly anticipated and will likely be the first novel agent approved for urothelial cancer in more than 20 years.
The FGFR inhibitors are also showing promise, with early phase I studies suggesting response rates as high as 40% to 50% in patients with FGFR3 mutations or translocations. Although FGFR mutations and translocations are predicted to occur in less than 20% of muscle-invasive bladder cancers, approvals of such agents may also play a role in earlier stage disease where the presence of these mutations maybe as high as 50%. Trials are currently accruing in the second-line setting in hopes of obtaining FDA approval.
Angiogenesis inhibitors still remain of interest in the treatment of urothelial cancer. Early studies suggested that overexpression of VEGF was associated with adverse outcomes and poor prognosis in patients treated with neoadjuvant chemotherapy (18). One mouse model suggested that these effects could be enhanced when angiogenesis inhibitors are combined with taxanes (30). Preliminary results of a randomized phase II trial of docetaxel plus or minus the VEGF inhibitor ramucirumab suggested an improvement in progression-free survival for the combination as a second-line therapy for metastatic urothelial carcinoma (31). A frontline trial of gemcitabine with cisplatin plus or minus bevacizumab is still completing accrual. Phase II results were promising, with an objective response rate of 72% and a median overall survival (OS) of 19.1 months (32). In the neoadjuvant setting, a trial of dose-dense M-VAC) with bevacizumab showed pT0N0 rates of 39%, with 5-year OS and disease-specific survival rates of 63% and 64%, respectively (33). Using GEP to molecularly characterize the tumors from this trial. we found that the 5-year survival for the basal subtype was 91% (in press, Evr. Urol.). Because the angiogenic signature is enriched in the basal subtype, one would hypothesize that to promote personalized medicine in urothelial cancer, future studies should focus on enrichment of basal tumors on clinical trials of antiangiogenic therapies.
In the United States, approximately 90% of all urothelial malignancies are within the histologic spectrum of transitional cell carcinoma (TCC), which, in our view, merges without obvious demarcation with the sarcomatoid and small cell variants at the extreme of dedifferentiation. Most of the remainder exhibit squamous histology (especially prominent in the more distal urethra) or glandular differentiation (ie, adenocarcinoma). The finding of adenocarcinoma apart from the context of bladder exstrophy or a urachal tumor (see below) should prompt consideration of metastatic disease from some other primary site because bona fide primary adenocarcinomas originating from the urothelium are distinctly uncommon.
As noted earlier, primarily hyperplastic lesions, biologically akin to polyps in the gastrointestinal tract, account for a large portion of incident cases. In 1999, the World Health Organization (WHO) put forward a new classification of noninvasive papillary urothelial tumors in recognition of the fact that the malignant potential of these lesions varies widely (34). The WHO terminology for these lesions, which together account for more than half of all new cases of urothelial neoplasia, is as follows:
Papillary urothelial neoplasm of low malignant potential. This lesion is characterized by orderly progression of morphology within the urothelium and no cellular atypia. Recurrence is seen in about 25% of cases, but progression is rare.
Noninvasive papillary urothelial carcinoma, low grade. This lesion shows some architectural variation and mild atypia. Such lesions commonly recur (in 50% or more of cases), but progression is seen in only 5% to 10%. It is classified by the WHO as a borderline tumor.
Noninvasive papillary urothelial carcinoma, high grade. This lesion shows predominantly disorganized architecture and moderate to marked cytologic atypia. Such lesions do not invade below the basement membrane, but they have substantial biologic potential, with progression to invasive, potentially life-threatening disease in up to 65% of cases. These are classified by the WHO as malignant. Note that in the WHO system, grades are collapsed to a two-tier system of low grade and high grade. In general, this would map to the older three-tier system as follows: grade 1 = low grade and grades 2 and 3 = high grade.
In contrast to these papillary cancers, some urothelial cancers exhibit dysplasia and chromosomal instability early on and constitute a “second pathway” of carcinogenesis (16,35,36), and most of the cases with lethal potential are in this class (see Fig. 36-1). In contrast to the classic “mulberry” appearance, the nonpapillary lesions have a grossly flat or infiltrative appearance at cystoscopy. In older literature, such cancers have been known as flat, sessile, solid, or tentacular. Currently, the preferred nomenclature is simply nonpapillary, although flat persists in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (seventh edition). These morphologic differences were noted long before an understanding of the genetic changes associated with cancer was appreciated. As expected for such distinct phenotypes, the characteristic genetic lesions have been found to be distinct.
In addition to papillary and nonpapillary variants, a micropapillary pattern is increasingly recognized (37,38,39,40). The term micropapillary originally came from the recognition of morphologic resemblance to an aggressive variant of ovarian cancer. Indeed, this general histologic pattern has been reported in many epithelial malignancies and invariably identifies a more aggressive subset, as was first reported for bladder cancer in 1994. Thus, the biologic potential of urothelial neoplasia extends from relatively nonthreatening in papillary lesions, to potentially life-threatening in nonpapillary lesions, to remarkably aggressive micropapillary lesions, all of which are recognizable as TCC.
Depending on the series, about 30% (or more) of muscle-invasive urothelial cancers are found to have focal areas of squamous or glandular morphology when examined in detail. It is not clear that such “mixed” tumors have a different prognosis than “pure” TCC, and we do not consider this a meaningful subset at University of Texas MD Anderson Cancer Center (MDACC). Certainly, tumors with only focal areas of squamous or glandular differentiation do not exhibit the distinctive natural history of pure squamous cancers or pure adenocarcinomas. Primary nonepithelial cancers (eg, sarcomas, lymphomas, melanomas) are exceedingly uncommon in the bladder. When they do occur, they do not appear to have a distinctive natural history or clinical management from what would be expected of similar tumors arising in more typical sites.
Urothelial cancers can dedifferentiate to include spindled (“sarcomatoid”), small cell, and plasmacytoid variants. In these cases, the clinical expression of the overall disease process is dominated by the aggressive component, even if most of the primary cancer is well within the typical morphologic spectrum of TCC.
Small cell carcinoma of the urothelium is a remarkably aggressive malignancy and exhibits a similar propensity for spread to the brain as small cell carcinoma arising in other sites. Even in the setting of clinically localized disease, the prognosis with local therapy alone remains poor, reflecting the early development of micrometastases. We strongly favor management of patients with cT2 or lower disease that is based on neoadjuvant chemotherapy, followed by surgical consolidation (41,42). In our hands, this provides about 70% cure (42,43). Patients with locally advanced disease have a high incidence (8 of 16 patients in our series) of relapse in the brain (43), and thus, we feel these patients are candidates for prophylactic cranial irradiation. Patients with metastatic disease at presentation continue to have nearly a 100% response rate, with a 100% relapse rate and a median survival of only 11 months (43).
Plasmacytoid urothelial cancer is a rare histology with limited series reported to date (44). These tumors typically appear mesenchymal with downregulation of E-cadherin (45,46) and consist of poorly cohesive tumor cells with perinuclear clearing reminiscent of plasma cells (46,47). They also tend to be highly proliferative with abundant mitosis and staining for Ki-67. These tumors tend to have a unique spread pattern, often appearing as a linitis plastica with diffuse thickening of the bladder and rectum. Although plasmacytoid tumors appear to be sensitive to chemotherapy, even in the neoadjuvant setting with adequate downstaging to pathologic T0 disease, there are few long-term survivors (44). These tumors have also been shown to have a high incidence of recurrence in the peritoneum (>50% of patients) (44).
Cancers arising in a urachal remnant, although not strictly “bladder cancers” in the sense of this chapter, merit a comment. These cancers typically involve the dome of the bladder and histologically are enteric-type adenocarcinomas. They are thought to reflect malignant transformation of an enteric epithelial rest within the urachal remnant, producing a cancer readily recognized as a mucinous adenocarcinoma (48). An early MDACC series suggested that the risk of relapse was increased when an en bloc resection was not performed and in the setting of positive margins, lymph node involvement, and tumor involving the peritoneal surface (49). The Memorial Sloan-Kettering Cancer Center experience in 50 patients demonstrated long-term survival in 26 (93%) of 28 patients with pathologically localized disease, in 9 (69%) of 13 patients with extension through the bladder or urachal cavity, and in none of 9 patients with peritoneal involvement initially (50). Similarly, the Mayo Clinic experience (51) in 49 patients demonstrated apparent cure in the majority of patients with confined disease and relapse in the majority of patients with nonconfined disease, with the latter group having a median survival of only 16 months (52). All investigators have emphasized the importance of an attempt at margin-negative, en bloc resection if at all possible.
Urachal cancers have a tendency to recur locally, often with peritoneal carcinomatosis, and the typical metastatic sites are (about equally) the lungs and liver. Although dramatic responses are infrequent, the use of modern combination regimens with activity in enteric adenocarcinoma is associated with about a 40% objective response rate. Thus, in essentially every clinical respect, urachal cancer behaves like colon cancer and, based on the available data, should be treated accordingly. Median survival from the recognition of metastases was 24 months in our retrospective series of 26 patients with metastatic disease, although some patients with grossly metastatic disease have been long-term survivors (49).
DIAGNOSIS, STAGING, AND PROGNOSIS
About 80% of patients present with hematuria, usually painless. The “typical” patient is a smoker in his late 60s. Such patients frequently suffer from pulmonary disease and cardiovascular disease, magnifying the morbidity of both chemotherapy and surgery. They are also at high risk for other smoking-related malignancies. A high percentage of patients have diminished renal function as a result of hypertension and obstructive nephropathy. Thus, the use of nephrotoxic chemotherapy is especially challenging in this population. Hospitalization and meticulous attention to detail are typically required to safely deliver multiple cycles of cisplatin- or ifosfamide-based therapy.
Irritative voiding symptoms, including frequency, dysuria, and dribbling, are important points in the medical history because the presence of irritative symptoms raises the possibility of extensive carcinoma in situ (CIS) or large, infiltrative tumors that may be far more extensive than is revealed by the initial cystoscopy. Obstructive symptoms (nocturia, double voiding, overflow incontinence, low anterior abdominal pain) are often encountered from tumors of the bladder neck or prostatic urethra. Tumors in these locations are far less likely to be anatomically confined (ie, curable with surgery) than are similarly muscle-invasive bladder cancers that are well away from these areas where the detrusor muscle is discontinuous.
In evaluating patients presenting with hematuria, it is mandatory to evaluate the upper urinary tracts. Even if a bladder tumor is confirmed on initial cystoscopy, excretory urography is still appropriate because synchronous or metachronous tumors of the upper tracts typically are not associated with specific symptoms.
Recently, several urine tests for cancer-associated antigens have been promulgated for diagnosis. None of these tests is yet sensitive and specific enough to replace cystoscopy and biopsy. The role of these tests for widespread screening is also not defined. Likewise, the role of urine cytology in initial diagnosis is unsettled. The yield is highly dependent on sample collection technique and the skill of the cytopathologist. As with many epithelial cancers, it is likely that DNA-based tests will eventually be widely used to find pathognomonic genetic alterations in urine and thus revolutionize the detection and clinical follow-up of bladder cancer. The role of some form of surveillance in high-risk populations (such as petrochemical workers) remains controversial. The relatively low positive and negative predictive value of cytology, even when applied to a high-risk group, makes such screening difficult to justify.
Excepting the extremes of histology (eg, small cell), the dominant prognostic clinical variable is anatomic stage at diagnosis. Classically, this is defined by depth of invasion. The currently used staging system is summarized in Fig. 36-3. This system is, of course, historically rooted in pathologic findings related to cystectomy specimens. As a result, it is not well suited for clinical
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree