It is difficult to reconcile what is known about the carcinogenic actions of asbestos and the long latency period between asbestos exposure and mesothelioma development. Lanphear and Buncher23 reviewed 21 studies of 1,690 mesotheliomas and found that 96% occurred at least 20 years after exposure; the mean latency was 32 years. Several theories have been postulated to describe what happens during this latency period.24 It is widely believed that asbestos induces genetic alterations in mesothelial cells over a long period that eventually lead to malignant cells. This may occur if a key regulatory gene, such as the INK4a/ARF locus, is deleted or silenced. With loss of key regulatory genes, additional mutations could accumulate rapidly. INK4a/ARF codes for p16 (a cyclin-dependent kinase inhibitor) and for p14ARF, which promotes murine double minute-2 degradation, preventing murine double minute-2 from neutralizing p53. Both p16 and p14ARF are often deleted in MM (see “Alterations of Specific Tumor Suppressor Genes in Mesothelioma”). This process may allow enough mutations to accumulate to lead to malignancy. Cell growth from this point would be rapid, leading to a clinically detectable tumor.1
OVERVIEW OF MOLECULAR MECHANISMS IN MESOTHELIOMA
Cytogenetic Assessment of Malignant Mesotheliomas
Deletions of specific chromosomal sites in the short (p) arms of chromosomes 1, 3, and 9, and long (q) arm of 6 are repeatedly observed in these tumors. Using newer molecular platforms including array comparative genomic hybridization and representational oligonucleotide microarray analysis, deletions in chromosomes 22q12.2, 19q13.32, and 17p13.1 appear to be the most frequent events (55% to 74%), followed by deletions in 1p, 9p, 9q, 4p, and 3p, and gains in 5p, 18q, 8q, and 17q (23% to 55%). Increasing numbers of copy number abnormalities in mesothelioma are associated with poor prognosis, especially when associated with deletions in 9p21.3 encompassing CDKN2A/ARF and CDKN2B, as detailed later.24 Analysis of the minimal common areas of frequent gains and losses has pointed to novel potential tumor suppressor genes (TSG) including OSM (22q12.2), FUS1, PL6 (3p21.3), DNAJA1 (9p21.1), and CDH2 (18q11.2–q12.3).25
Alterations of Specific Tumor Suppressor Genes in Mesothelioma
p16
Cheng et al.26 reported homozygous deletions of one or more of the three p16 exons in 34 of 40 (85%) malignant mesothelioma cell lines and a point mutation in one cell line. Downregulation of p16 was observed in four of the remaining cell lines. Homozygous deletions of p16 were identified in 5 of 23 (22%) malignant mesothelioma tumor samples. Downregulation of p16 in malignant mesothelioma cells may result from 5′CpG island hypermethylation, as has been demonstrated in other types of cancer. P16 inactivation by homozygous deletions or methylation is a frequent event in Japanese patients with malignant pleural mesotheliomas (MPM).27 Homozygous deletion is the major cause of the P16 inactivation,28 but methylation also leads to the inactivation of P16 when the P16 alleles are retained.27 Homozygous deletions at this locus can lead to the inactivation of another putative TSG, p14ARF, because p16 and p14ARF share exons 2 and 3 although their reading frames differ.29,30 The prognostic significance of p16/CDKN2A loss in MM has been defined both by immunohistochemistry and fluorescence in situ hybridization (FISH). With the FISH analysis of primary MM tissue samples or MM cells from the pleural effusion, over 70% of cases showed homozygous deletions of the CDKN2A/ARF locus. Both loss of p16 protein expression by immunohistochemistry and homozygous deletion of p16 by FISH are associated with an adverse prognosis.31 Loss of p15 is also associated with codeletion of microRNA (miR)-31.32 Protein phosphatase (PPP6C), upregulated in MPM, is a target of mir-31, and reintroduction of miR-31 in mesothelioma cells has a suppressive effect on MM cells.32
p53
Cell lines derived from murine models of asbestos-induced mesothelioma have reduced or absent expression of p53 messenger RNA (mRNA) compared to the RNA from nontumorigenic cell lines or reactive mesothelial cells.33 In human cell lines derived from patients with malignant mesothelioma, attention has turned to specific mutations or genetic abnormalities in p53. In general, mutational analyses of p53 in mesothelioma have not revealed reasons for its inactivation.
Wilms Tumor Gene
The detection of Wilms tumor gene (WT1) mRNA or protein provides a specific molecular or immunohistochemical marker for differentiation of mesothelioma from other pleural tumors, in particular adenocarcinoma.34 Vaccination protocols that exploit the selective presence of WT1 in mesothelioma are under way at selected institutions.35
Neurofibromatosis Gene
The tumor suppressor merlin is encoded by the neurofibromatosis type 2 gene (NF2), which is located on chromosome 22q12, and mutations in this gene have been found in 40% of mesothelioma and 50% of malignant mesothelioma cell lines.36,37 Mutations including deletions and insertions lead to truncated and inactivated merlin. Experimental animal models indicate that disruption of the NF2 signaling pathway, together with a deficiency in ink4a, is essential for mesothelioma development.38
ALTERATIONS OF ONCOGENES IN MESOTHELIOMA
C-sis (Platelet-Derived Growth Factor)
Malignant mesothelioma cell lines produce numerous growth factors and cytokines, possibly as a consequence of altered oncogene status. One of the more intriguing oncogenes in malignant mesothelioma is C-sis, which codes for one of the two chains (alpha and beta) of platelet-derived growth factor (PDGF). Gerwin et al.39 were the first to describe elevation of RNA levels for both chains of PDGF in mesothelioma cell lines and correlated the increase with PDGF-like activity secreted by the cells. It is of interest that overexpression of A chain transforms human mesothelial cells to the tumorigenic phenotype in vitro and that the use of antisense ODN to the A chain inhibits growth of mesothelioma cell lines. PDGF-D promotes mesothelioma cell proliferation by targeting ROCK or MAP kinase through autocrine activation of PDGF-beta receptor40; however, in general, therapies targeting PDGF in mesothelioma have been unsuccessful.41
Transforming Growth Factor
Transforming growth factor-B (TGF-B) is another growth-regulatory and immunomodulatory cytokine, and high levels of TGF-B have been described in several human and mouse malignant mesothelioma cell lines.42,43 TGF-B may have a potential role in regulation of the differential PDGF receptor expression in malignant mesothelioma and thus have an effect on proliferation as well as other events. TGF-B production by tumors plays a significant role in blocking immune response by preventing T-cell infiltration into tumors, inhibition of T-cell activation/function, and mediation of T regulatory cell–induced immunosuppression. TGF-blockers (soluble receptors/antibodies) and receptor inhibitors have antitumor effects that, in several models, are due primarily to immunologic mechanisms. TGF blockade had antitumor effects that were CD8+ T-cell dependent in a murine malignant mesothelioma model.44 Recently, it was reported that a combined triple treatment of anti-CD25 monoclonal antibody, anti–cytotoxic T-lymphocyte antigen-4 monoclonal antibody and TGF-beta soluble receptor resulted in long-term clearance of MPM tumors and memory against tumor rechallenge.45
Insulin Growth Factor
Insulin growth factor (IGF) is another of the important autocrine loops in mesothelioma.46 The IGF receptor-1 pathway is a significant regulator of mesothelioma growth acting through downstream kinases such as Akt. In humans, both normal mesothelium and mesothelioma cell lines express IGF-1 and IGF-1R RNAs (by Northern blot or reverse transcriptase polymerase chain reaction), and IGF-1 protein is detected in their conditioned media. These studies suggest that mesothelioma growth, both in human and animal models for the disease, may involve the IGFs, and trials using anti-IGF-1R antibodies or small-molecule IGF blockade are being formulated for mesothelioma.47 Cixutumumab, a humanized monoclonal antibody to IGF-IR, has been investigated using early passage tumor cells and an in vivo human mesothelioma tumor xenograft model. Cixutumumab induced antibody-dependent cell-mediated toxicity and in vivo, cixutumumab treatment delayed growth of H226 mesothelioma tumor xenografts in mice and improved the overall survival of these mice compared to controls.48 A phase 2 clinical trial of cixutumumab is currently ongoing for the treatment of patients with mesothelioma.
Angiogenic Mechanisms in Mesothelioma
The most relevant cytokines and growth factors in angiogenic pathways involve the ILs (specifically IL-6 and IL-8), fibroblast growth factors (FGF), vascular endothelial growth factors (VEGF), platelet-derived endothelial growth factors, and IGFs. Moreover, mutational or posttranslational silencing of TSGs or their products, particularly p53 and p16, has also been associated with increased angiogenic mechanisms.
IL-6 levels are elevated in the serum and pleural effusions of patients with mesothelioma, and there have been correlations seen between the IL-6 level and the degree of thrombocytosis in these patients.49 IL-6 expression is elevated in tissues undergoing angiogenesis, but by itself it has a limited role in proliferation of endothelial cells. IL-6, however, induces angiogenesis by elevating the expression of VEGF, a specific mitogen for vascular endothelial cells. Serum VEGF and IL-6 levels are elevated in patients with mesothelioma, and their levels each correlate with platelet count.50 IL-8 is also an important angiogenic factor for the development of new capillaries in vivo and has direct growth-potentiating activity in mesothelioma. Pleural fluid from patients with mesothelioma has significantly higher IL-8 levels by enzyme-linked assay compared to patients with other malignant effusions.51 Mesotheliomas present with a high intratumoral vessel density that also have prognostic relevance,42,52,53 and immunohistochemically, VEGF, FGF-1 and -2, and TGF-B immunoreactivity are present in 81%, 67%, 92%, and 96%, respectively, of mesotheliomas, and in 20%, 50%, 40%, and 10%, respectively, of samples of the nonneoplastic mesothelium. Depending on the study, high immunohistochemical FGF-2 expression53 or VEGF expression by reverse transcriptase polymerase chain reaction correlate with more tumor aggressiveness and worse prognosis for mesothelioma.52,54
Simian Virus 40
SV40 is a DNA tumor virus that has been associated with the development of malignant mesothelioma.55 Although this virus is endogenous in rhesus monkeys, epidemiologic studies have shown that it is also widespread among the human population. The mode by which the virus was transferred from monkey to human is uncertain, but the bulk of this transfer may have occurred from 1954 to 1963 through SV40-contaminated polio vaccines administered worldwide. Moreover, polio vaccines produced in the former Union of Soviet Socialist Republics remained contaminated with infectious SV40 at least until 1978.56
The association of SV40 with mesothelioma started with the observation that when hamsters were injected with SV40 into the pleural space, all of the animals developed mesotheliomas within 6 months. This finding prompted investigations into the possibility that some mesotheliomas in humans could be attributed to SV40 infection directly or with SV40 acting as a cocarcinogen with asbestos. Mesothelioma samples studied in 1994 showed that 60% of the samples contained SV40 DNA and expressed the SV40 large T antigen. The results were confirmed by numerous laboratories using a variety of techniques such as PCR, FISH, Western blot, immunohistochemistry, and laser dissection/PCR, but the percentage of positive samples varied from 6% to 83%, and a few studies were completely negative. Technical and geographical differences may account for these variances.
Although the epidemiologic data are not available, mechanistic experiments in human mesothelial cells57,58 and animal experiments59 strongly support a pathogenic role of SV40 in mesothelioma. It is unlikely, however, that SV40 acts alone in mesothelioma development, as most cancers are multifactorial, and most mesotheliomas occur in asbestos-exposed individuals. SV40 has been shown to be a cocarcinogen in causing mesothelioma in animals and malignant transformation of mesothelial cells in tissue culture.60,61 It is safe to say that a definitive role for the virus in human mesothelioma has not been unequivocally demonstrated but ongoing debate regarding its role will continue.
Radiation and Mesothelioma
In the literature, there are a few studies reporting mesothelioma development in patients exposed to thorotrast (intravenously) or who had received radiation to the chest and abdomen.1 In some of these cases, asbestos exposure could not be ruled out, and SV40 status was not investigated. However, in a few cases in which mesotheliomas developed in young adults who received radiotherapy (RT) because of Wilms tumor, radiation was the only likely causative factor. Studies in rats support a role for radiation as a causative factor. Although it appears that radiation may cause mesotheliomas, the number of mesotheliomas for which it is responsible is probably small.
Carbon Nanoparticles
Engineered single-wall carbon nanotubes (SWCNT) are a class of nanoparticles being actively evaluated for myriad industrial and biomedical applications.62 Preliminary cellular and animal exposure investigations on the toxicity and pathogenicity of SWCNTs have demonstrated biologic interactions, including toxicity, inflammatory reactions, oxidative stress, and fibroproliferative response.63–65 SWCNTs are biopersistent and have the ability to distribute to subpleural areas after pharyngeal aspiration. Reports have described the induction of mesothelioma in p53+/− mice by multiwall carbon nanotubes.66,67 These investigations compelled the present studies of potential interactions of SWCNTs with mesothelial cells. Exposure to SWCNTs induced reactive oxygen species generation, increased cell death, enhanced DNA damage and H2AX phosphorylation, and activated poly(adenosine diphosphate-ribose) polymerase, AP-1, NFκB, p38, and Akt in a dose-dependent manner. These events recapitulate some of the key molecular events involved in mesothelioma development associated with asbestos exposure.68,69 In addition, nickel nanoparticles enhance the in vitro activity of PDGF in regulating chemokine production in rat mesothelial cells through a mechanism involving reactive oxygen species generation and prolonged activation of ERK-1.70
Genetic Predisposition to Mesothelioma: Erionite and BAP1
Recent evidence indicates that genetic predisposition plays an important role in determining individual susceptibility to mineral fiber carcinogenesis and to the development of mesothelioma. In the villages of Karain (population ~600), Tuzkoy (population ~1,400), and Sarihidir (this village was abandoned) in Cappadocia, a region in Central Anatolia, Turkey, ≥50% of deaths are caused by malignant mesothelioma.71 This epidemic has been linked to exposure to a mineral fiber called erionite, one of the most potent mineral fibers in causing mesothelioma. However, mesothelioma was more frequent in certain families than others, leading to observations that some families in Turkey were unusually susceptible to erionite carcinogenesis.71 Erionite is present in many parts of the world, including western American states such as North Dakota,72,73 but so far the contribution of erionite to the increased incidence of mesothelioma has not been studied except in Turkey.
Similar families have also been identified in other parts of the world74 and in the United States, where they appear to be unusually susceptible to asbestos carcinogenesis. Recently, BRCA-associated protein 1 (BAP1) has been identified as a novel MPM TSG. BAP1 is located at the 3p21, a region frequently deleted in MM, and encodes for a deubiquitinase enzyme known to target histones and other proteins. BAP1 appears to exert its antitumor activities mainly in a BRCA-independent manner,75 with regulation of the cell epigenome, via modulation of histone H2A ubiquitination and chromatin accessibility.76 However, the relevance of BAP1 to the biology of normal and cancer cells remains largely unexplained; in fact, manipulation of BAP1 in cancer cells has often yielded unexpected or even contradictory results. For example, silencing of BAP1 in MM and uveal melanoma cell lines resulted in reduced cell growth.77,78
Bott et al.77 originally described the association of somatic mutations of BAP1 in 23% of mesothelioma samples. In a larger series of patients, the same group confirmed that somatic BAP1 mutations occur in about 20% of pleural MM and reported that the only clinical variable significantly different among those with and without BAP1 mutations was smoking (former or current), with BAP1 mutations more prevalent among smokers (75% versus 42%).79 A Japanese study reported BAP1 gene alterations (either deletions or sequence-level mutations) in 61% of their 23 MM samples.80 Whether this discrepancy results from the different methodologies in sample preparation and detection of BAP1 mutations or it is an intrinsic difference between the two populations (e.g., due to ethnicity) has still to be determined. A third recent study from Arzt et al.,81 with a separate cohort of 52 pleural MM, reported absence of BAP1 IHC staining in 60% of pleural MM, confirming previous results. Moreover, there was no correlation between BAP1 expression and asbestos exposure, and it was suggested that expression of BAP1 in tumor samples is inversely correlated to survival.
Germline BAP1 mutations were reported by Testa and Carbone82 in two US families having a high frequency of MPMs. In the same study, 22% sporadic MM tumors harbored somatic BAP1 mutations. These findings have promoted speculation that BAP1 germline mutations can cause a novel cancer syndrome characterized by a significant excess of both pleural and peritoneal MM, uveal and cutaneous melanoma, and possibly other tumors.
Benign Mesotheliomas
There are a number of benign mesothelial proliferations that must be distinguished from malignant mesothelioma including multicystic mesothelioma (multilocular peritoneal inclusion cyst), adenomatoid mesotheliomas, and well-differentiated papillary mesothelioma. Localized mesothelioma, better referred to as localized fibrous tumor of the pleura (FTP), is similar to other fibrous tumors found elsewhere in the body. The tumor cells have a benign appearance and are usually immersed in a fibrous and characteristically vascular stroma. FTPs are characteristically negative for cytokeratin (a marker of mesothelial cells) and positive for CD34, which suggests that these cells are not of mesothelial origin. The most important predictive factor in the prognosis of FTP is whether the tumor can be completely resected. Pedunculated tumors have a much better prognosis than tumors that grow over a broad pleural area.83–85 Surgical resection (Fig. 114.2) is the treatment of choice, with complete resection of the tumor and its pedunculated portion along with the site of origin. Five-year survival rates as high as 97% have been reported. En bloc resection of the lung, chest wall, or diaphragm may be necessary. For recurrences, re-excision is the treatment of choice. Long-term survival with re-excision is possible, but with incomplete resection or malignant transformation the median survival is 24 to 36 months.

Malignant Mesothelioma
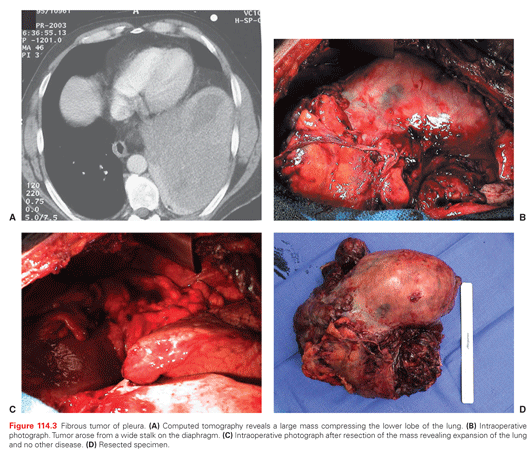
Histologically, malignant mesothelioma can show an epithelial morphology (malignant mesothelioma epithelial type), a fibrous morphology (malignant mesothelioma fibrous type, also called sarcomatoid type), or a combination of both (mixed type or biphasic malignant mesothelioma) (Fig. 114.3). Most MM (50% to 60%) are of the epithelial type, approximately 10% are sarcomatoid, and the remainder are biphasic MM.
The diagnosis of malignant mesothelioma is usually straightforward, in contrast to common belief, provided that the pathologist has extensive experience with this malignancy. Some epithelial mesotheliomas grow forming sheets of epithelioid cells. To rule out mimics of mesothelioma, the diagnosis should be confirmed by immunohistochemistry, which shows that the tumor cells are diffusively positive for pankeratin, keratin 5/6, calretinin, and WT-1, and negative for the epithelial markers such as CEA, CD15, Ber-EP4, Moc-31, TTF-1, and B72.3. For epithelial mesotheliomas, positive stainings for pankeratin and calretinin and negative stainings for three epithelial markers are considered sufficient for diagnosis.
Biphasic mesotheliomas can be distinguished from carcinosarcomas metastasizing from various organs and from biphasic synovial sarcomas by using either electron microscopy or molecular testing for the X;18 translocation that is diagnostic of synovial sarcoma.86
Newer methods are under development for distinguishing mesothelioma from adenocarcinoma and include microRNA profiling of the tumors. Gee et al.87 have described the use of seven miRNAs (miR-200c, miR-141, miR-200b, miR-200a, miR-429, miR-203, and miR-205) to distinguish lung adenocarcinoma from mesothelioma with a 10% misclassification error. Others have described a three microRNA profile (mir-205, mir-193a-3p, and mir-192) for the differential diagnosis of mesothelioma from colon, renal, lung, and breast cancer with a 95% specificity and 96% sensitivity.88
EPIDEMIOLOGY AND CLINICAL PRESENTATION
The death rates for mesothelioma parallel the consumption of asbestos by individual countries. When one considers mesothelioma death rates from 2000 to 2004 as a function of asbestos usage in the 1960s, Australia has the highest death rate for the disease in the world.89 It is predicted that between 2000 and 2049, over 400,000 lives will be lost in those countries from the disease.90 In the United States, there have been 2,500 deaths per year from the disease since 1999, with workers with industrial exposures in construction and shipbuilding being at highest risk, followed by plumbers, pipe fitters, and steamers. Mesothelioma affects men in their 50s, 60s, and 70s due to the aforementioned 25- to 40-year latency period between occupational asbestos exposure and the development of the tumor.91 Women and children can have the disease, but the male-to-female ratio is approximately 4.5:1.92
Symptoms
As many as 25% of patients with the disease have symptoms for ≥6 months before seeking medical attention. The right side is affected more than the left side (60% versus 40%), most likely because of the right side’s greater volume.
Approximately 60% of the patients present with nonpleuritic chest pain that classically is located posterolaterally and low in the thorax. Dyspnea is present in 50% to 70% of the cases, and, indeed, 80% of the patients present with dyspnea and effusion. The presence of a pleural effusion is documented at some time in the course of the disease in 95% of patients with MPM. With the increasing use of positron emission tomography for pretreatment planning, between 5% and 25% of patients are found to have metastatic disease at presentation.93
Physical Examination
Physical examination reveals decreased breath sounds, dullness to percussion, or decreased motion of the involved chest wall. Failure to significantly relieve the dyspnea after thoracentesis may indicate that the lung is “trapped.” In the late stages of the disease, there is often dramatic cachexia, marked contraction of the involved chest with narrowed interspaces, and hypertrophy of the contralateral hemithorax. A chest wall mass occurs in up to 25% of patients, often at the site(s) of prior thoracentesis, thoracotomy, or thoracoscopy wounds.
Laboratory Examination and Blood Biomarkers
Mesothelioma patients have nonspecific laboratory findings, including hypergammaglobulinemia, eosinophilia, and/or anemia of chronic disease. It has been recently noted that 14% to 15% of patients have elevated homocysteine levels, reflecting folic acid deficiency; 17% have biochemical evidence of vitamin B12 deficiency; and 32% have biochemical signs of vitamin B6 deficiency.94 The most striking laboratory abnormality is thrombocytosis (>400,000), which is seen in 60% to 90% of patients,95 and approximately 15% of patients have platelet counts >1,000,000.
Soluble mesothelin-related peptide (SMRP), osteopontin (OPN), and megakaryocyte potentiating factor are presently under investigation as early detection or therapy monitoring markers. Robinson et al.96 reported that determination of SMRP in serum is a marker of mesothelioma with a sensitivity of 83% and specificity of 95% in the first 48 patients with MPM tested. These data have been validated by other laboratories in the United States97,98 and internationally.99 Changes in serum SMRP levels parallel clinical course/tumor size, and SMRP was elevated in 75% of patients at diagnosis.100 Studies using SMRP in an attempt to diagnose early mesothelioma in high-risk cohorts have so far been disappointing, but follow-up is still short.101,102
OPN is a glycoprotein that mediates cell-matrix interactions and is regulated by proteins in cell-signaling pathways that have been associated with asbestos-induced carcinogenesis.103 In a study that compared serum OPN levels of patients with MPM to those of asbestos-exposed individuals without cancer, serum OPN levels rose with duration of asbestos exposure and degree of radiographic abnormality (plaques and fibrosis versus other lesser findings104), and using a cutpoint of 48.3 ng of OPN per milliliter resulted in 77.6% sensitivity and 85.5% specificity when comparing the group exposed to asbestos with the group with mesothelioma. Follow-up studies have demonstrated that serum OPN is not reliable because of the presence of a thrombin cleavage site, which causes degradation of the marker.105 Plasma OPN,106 although not specific to mesothelioma, is being evaluated in the disease for both prognosis and early detection utility. A blinded validation of plasma fibulin 3 confirmed excellent discrimination between MPM plasma and all non-MPM cohorts including those with benign or malignant effusions. Pleural effusion fibulin 3 was also discriminatory between MPM effusions and non-MPM effusions.107
Radiologic Examination
Malignant mesothelioma can have a diverse radiographic appearance. Many of the early changes are associated with a previous exposure to asbestos, consisting both of pleural and parenchymal changes, including pleural plaques or parenchymal pulmonary fibrosis.
Chest Radiography
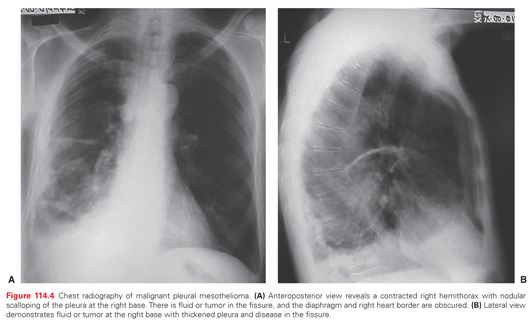
The most common chest radiography features associated with mesothelioma progression and symptoms include the presence of a pleural effusion, diffuse pleural thickening, and nodularity. The involved hemithorax can eventually have smooth, lobular pleural masses that infiltrate the pleural space and fissures108–110 in 45% to 60% of patients with contraction and fixation of the chest (Fig. 114.4). The lung becomes encased, and the mediastinum shifts because of volume loss.
Chest Tomography
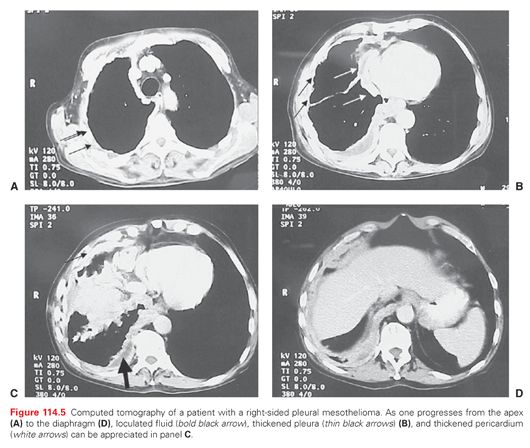
Pleural changes on chest tomography (CT) include pleural plaques, diffuse pleural thickening, and pleural effusion. Additional CT features of mesothelioma are localized nodular or plaque-like pleural thickening, possibly associated with pleural effusion. The lobulated pleural encasement frequently causes lower lobe collapse (Fig. 114.5). Intrapulmonary nodules can occur in 60% of patients, and infiltration into fissures along with enlarged hilar and mediastinal lymph nodes may be seen. The CT allows a better view of the involved pericardium, which is irregularly thickened and associated with infiltration to the pericardial fat pad. A clear fat plane between the inferior diaphragmatic surface and the adjacent abdominal organs as well as a smooth inferior diaphragmatic contour may imply resectability.111 CT may reveal a hemidiaphragm encased by a mass or poor definition between the liver, stomach, and inferior diaphragmatic surface.
Volumetric measurements using CT have recently been studied with regard to their prognostic capability (see “Prognostic Indicators”).
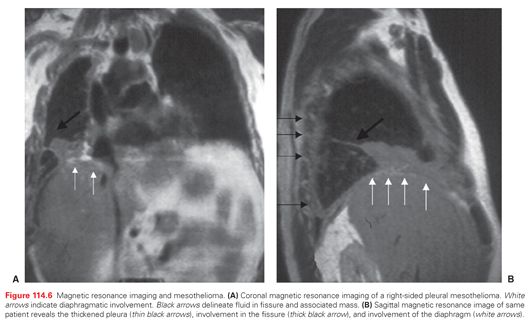
Magnetic Resonance Imaging
Studies have suggested that gadolinium contrast enhancement magnetic resonance imaging can improve mesothelioma staging (Fig. 114.6). Detection of diaphragm invasion and invasion of endothoracic fascia or a single chest wall focus may be better with magnetic resonance imaging compared with CT.111
Positron Emission Tomography Imaging
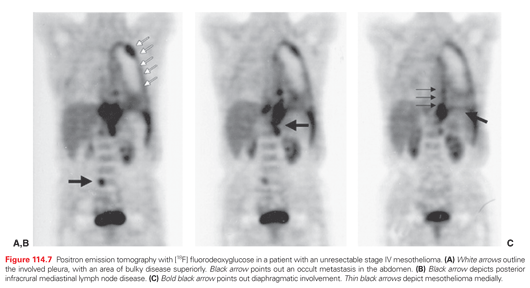
There are a number of studies of positron emission tomography (PET) and the radionuclide imaging agent [18F]fluorodeoxyglucose (FDG) in mesothelioma (Fig. 114.7). Early studies reported that FDG-PET was accurate in the diagnosis of pleural malignancies, specifically mesothelioma.112–116 Recent studies have concentrated on the accuracy of PET in defining nodal and extrathoracic metastases prior to surgical therapy.93,112 Because of false-positive results in patients with FDG-avid pleural inflammatory/infectious etiologies, all FDG-avid extrathoracic sites should be histologically confirmed in patients with MPM being considered for extrapleural pneumonectomy (EPP).
DIAGNOSTIC APPROACH FOR PRESUMED MESOTHELIOMA
Thoracentesis and Closed Pleural Biopsy
Patients who present with a large, unexplained pleural effusion and minimal or moderate evidence of pleural thickening should have initial thoracentesis and pleural biopsy, especially if a history of asbestos exposure or mesothelioma in a family member is elicited. By preserving a cell block from the pleural fluid and using both histochemical and immunohistochemical staining techniques along with electron microscopic analysis, the diagnosis of mesothelioma can be obtained from the pleural effusion in as high as 84% of suspected cases.
Thoracoscopy
A video-assisted thoracoscopy is indicated for patients at risk for mesothelioma who (1) develop a large effusion and who have negative studies on thoracentesis and pleural biopsy, or (2) recur with effusion after initial thoracentesis. Thoracoscopy can be invaluable for estimating extent of disease with regard to the lung, diaphragm, pericardium, chest wall, and nodes.117,118 Development of chest wall masses from seeding of the biopsy site or surgical scar is an uncommon complication (approximately 10%) of any diagnostic procedure, and the efficacy of radiotherapy to the scar to prevent recurrence is controversial.119–121
If there is no free pleural space because of previous treatment of pleural effusion and the bulk of the disease in the hemithorax is solid, open biopsy should be performed such that the scar can be incorporated into the incision at the time of cytoreduction surgery.
The majority of patients with treated or untreated pleural mesothelioma die of complications of local disease because of (1) increasing tumor bulk that eventually replaces the pleural effusion and causes progressive respiratory compromise, pneumonia, or myocardial dysfunction with arrhythmias; (2) unrelenting chest wall pain requiring narcotics, which leads to cachexia; and/or (3) dysphagia from tumor compression of the esophagus. Patients generally die of respiratory failure or pneumonia. Small bowel obstruction from direct extension through the diaphragm develops in approximately one-third of patients, and 10% die of pericardial or myocardial involvement.122 One of the most important clinical predictor of survival in noncytoreduced patients is performance status.95
Extrathoracic metastases occur late in the course of disease and are not usually the direct cause of the patient’s death. In the largest series of patients with MPM who had autopsy, 54% to 82% had distant metastases, with the most frequently involved organs being the liver, adrenal gland, kidney, and contralateral lung.123,124 Intracranial metastases are seen in approximately 3% of patients and are predominantly of the sarcomatous type.124,125
Prognostic Indicators
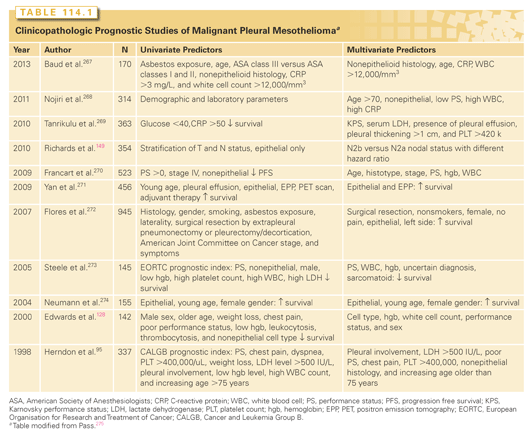
The best-known clinical prognostic scoring systems for MPM have originated from European Organisation for Research and Treatment of Cancer (EORTC) and Cancer and Leukemia Group B (CALGB), and use a combination of biologic and clinical factors. Poor performance status, nonepithelioid histology, male gender, low hemoglobin, high platelet count, high white blood cell count, and high lactate dehydrogenase were found to be poor prognostic indicators in mesothelioma.95,126 The EORTC model was validated at St. Bartholomew’s Hospital in sequential chemotherapy trials,127 and both models were validated by Edwards.128 Many other studies have explored clinical and pathologic features of MPM as seen in Table 114.1. Most recently, the ratio of absolute neutrophil to lymphocyte ratio was promising in segregation of risk for death129–131; however, a recent study found that the aforementioned EORTC and CALGB indices were superior.132
The molecular prognostication of mesothelioma has also been explored using gene expression array technology.133,134 There has, however, been variability in the gene sets and results of these prognostic tests when used in other MPM cohorts.135
The presence of a single microRNA, mir-29c*, has been associated with improved time to progression and overall survival but needs further validation.136 A variety of other tissue-based prognostic markers, including thymidylate synthase,137 OPN,138,139 and HAPLN1,140 have been reported but require validation.
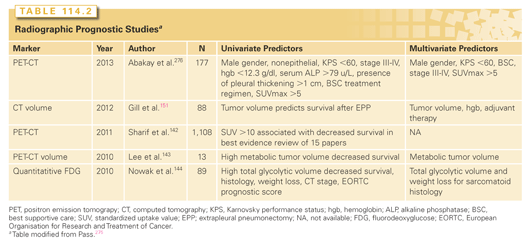
Quantification of the standardized uptake value (SUV) for PET scanning has also been investigated in mesothelioma as a prognostic marker (Table 114.2). Low SUV and epithelial histology predict the best survival, whereas high SUV and nonepithelial histology indicate the worst survival.112,141–144
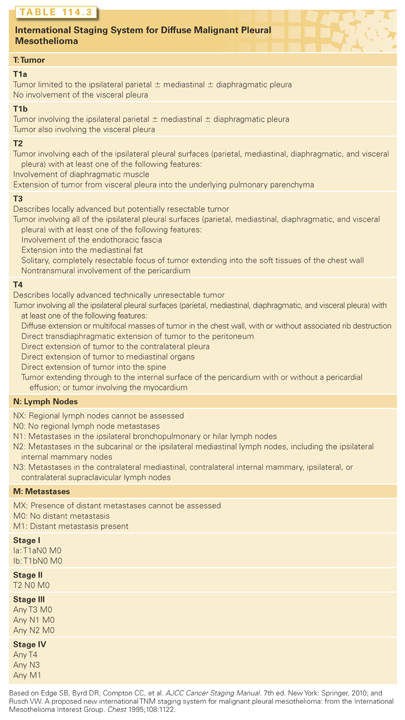
Staging
The original MPM staging system proposed by Butchart et al.145 relied on pathologic generalizations instead of specific quantitative aspects of the disease and was designed at a time that was well before recognition of different prognostic implications of N1 and N2 nodes. The American Joint Committee on Cancer staging system for mesothelioma (Table 114.3) was adopted from that proposed by the International Mesothelioma Interest Group (IMIG) in 1995146 and has been validated in a number of surgically based trials. In collaboration with IMIG, the International Staging and Prognostic Factors Committee of the International Association for the Study of Lung Cancer recently formed a Mesothelioma Domain in order to improve the current staging system resulting in the first large, international MPM database, which included over 2,000 staged patients with MPM diagnosed from 1995 to 2008 who had a surgical procedure. As described by Rusch et al.,147 a set of covariates were identified as predictive of survival in a “core” model for this analysis which included best staging information, age, sex, histology (epithelioid or not), and type of surgical procedure (palliative versus extrapleural pneumonectomy or pleurectomy decortication).147
Regrouping of the T and N combinations based on relative hazard and Kaplan-Meier survival analysis of 368 patients having EPP resulted in improved stage distribution (stage I–IV: 8%, 43%, 33%, 16%, respectively) and survival stratification (51 months, 26 months, 15 months, 8 months, respectively).148,149 A stage-independent model of risk for death in patients having surgical cytoreduction has expanded on the data from Pass et al.,150 which described the influence of tumor volume and survival in MPM. Gill et al.,151 in a study of 88 epithelial MPMs having EPP, reported that tumor volume, hemoglobin concentration, platelet count, pathologic TNM (tumor, node, metastasis) category, and administration of adjuvant chemotherapy or RT were associated with survival. In the final multivariate model, tumor volume, hemoglobin concentration, and administration of adjuvant chemotherapy or RT were identified as independently associated with overall survival.151 Richards et al.152 recently described a validated model for survival and time to recurrence in cytoreduced MPM using hemoglobin, CT volume, thickness of the fissures, and histologic subtype.
Overview
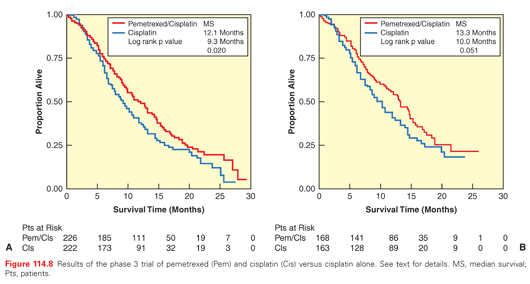
For unresectable patients who are candidates for chemotherapy, the well-powered phase 3 trial that showed a 3- to 4-month survival advantage for the two-drug regimen of pemetrexed and cisplatin compared with the one-drug regimen of cisplatin has defined the standard of care94 (Fig. 114.8). Recommendations for the management of pleural mesothelioma have been made by the European Society for Medical Oncology153 and the National Comprehensive Cancer Center Network.
Supportive Care
The median survival of patients with MPM who select supportive care ranges only from 4 months128 to 13 months.122 This variation is likely due to variations in tumor biology, host response to tumor, detection bias, lead-time bias, and the use of ad hoc or unreported treatments by some patients and physicians.
There are a number of options for the control of pleural effusion, including repeated thoracenteses, talc pleurodesis, or placement of a PleurX (Denver Biomedical, Denver, CO) catheter.154 Talc pleurodesis is performed by instilling 2 to 5 g of asbestos-free, sterile talc over the lung and the parietal surfaces. Success rates in effusion control with talc, used either via thoracoscopy or via slurry, approach 90%.
The MesoVats trial was a randomization between 2003 and 2012 of 87 video-assisted thoracoscopic pleurectomy or 88 talc pleurodesis. A total of 84% had epithelioid disease, 78% were IMIG stage ¾, and 49% were high risk as per EORTC criteria. Although there was no difference in survival between the two groups, pleural effusion was better controlled with patients who underwent pleurectomy at 1 month and 6 months. Moreover, there was a significant benefit in quality of life at 6 months and 12 months in favor of the pleurectomy group.155 Failure of these techniques is usually associated with lung trapped by the tumor, a large solid tumor mass, a long history of effusion with multiple thoracenteses leading to loculations, age older than 70 years, or poor performance status. In such cases, the PleurX catheter with its one-way valve can be implanted under local anesthesia into pleural effusion, and patients can drain themselves at home using the available disposable drainage kits.156
The chest pain of malignant mesothelioma requires narcotics, and these patients should be seen in consultation by a dedicated pain management team to assist in the optimization of the patient’s quality of life. Subcutaneous tunneling of epidural catheters for long-term out-of-hospital use has also been used in selective cases. When only local RT has been delivered solely for chest wall pain or chest wall nodules, the median survival has been reported to be 4 to 5 months.157,158
SURGERY FOR PLEURAL MESOTHELIOMA
The Controversy
Surgery for pleural mesothelioma remains a controversial subject not only among medical oncologists, but with thoracic surgeons. Much of the controversy remains as the result of the early trials of EPP with reported mortality rates of 31%,159 as well as the lack of systemic therapies besides pemetrexed and cisplatinum with response rates >30%, which can complement present surgical therapy with mortality rates of 4%. Despite strict functional criteria for the selection of patients to undergo mesothelioma surgery, there are no reliable prognostic predictors to stratify patients by the biology of the disease in order to determine which patients will recur rapidly after cytoreductive surgery. As discussed in the following, the type of operation to be performed (EPP or some sort of lung sparing pleurectomy with decortication (P/D) is one of the more debatable subjects for the management of the disease, and at present, suffers from the lack of randomized data that defines the present cadre of surgical interventions in a uniform manner. These issues, as well as the interpretation of high profile recently completed trials, have led to geographical variation in physicians’ use of surgery as part of the management plan.160 In a questionnaire answered by 802 thoracic surgeons belonging to The European Association for Cardio-Thoracic Surgery, The European Society of Thoracic Surgeons, or the Society of Thoracic Surgeons, EPP was believed to be more effective than P/D and the addition of adjuvant chemotherapy and multimodality therapy were believed to increase the chance of cure (see the following). American surgeons, however, were twice as likely to believe that EPP alone can cure mesothelioma as those in Europe (45% versus 23%).
The Purpose of Surgery
Operation for malignant pleural mesothelioma can be done simply for diagnostic or palliative purposes, but in selected patients, it is performed as a maximal cytoreductive procedure in which the persistent disease at the end of the operation is microscopic.
Diagnostic Procedures
In patients with localized or free flowing pleural effusion suspected of having mesothelioma, video thoracoscopy allows for definitive diagnosis. For subtype classification of mesothelioma histology, biopsy by open pleural biopsy or thoracotomy was found to be the most accurate in subtype classification (83%) compared with thoracoscopy (74%) and imaging-guided core or needle biopsy (44%) in a study comparing histologic subtype recorded by pre-EPP diagnostic method to the EPP surgical specimen.161,162
Maximal Cytoreductive Procedures
The goal of cytoreductive surgery in MPM should be the removal of all visible or palpable tumor (R0 or R1) or a “macroscopic complete resection” (MCR). MCR should be the goal of cytoreductive surgery, regardless of whether that involves EPP or a lung-preserving operation. The Mesothelioma Domain of the International Association for the Study of Lung Cancer has recommended the following as uniform nomenclature for pleural mesothelioma resections.163
EPP: en bloc resection of the parietal and visceral pleura with the ipsilateral lung, pericardium, and diaphragm. In cases where the pericardium and/or diaphragm are not involved by tumor, these structures may be left intact (Fig. 114.9).
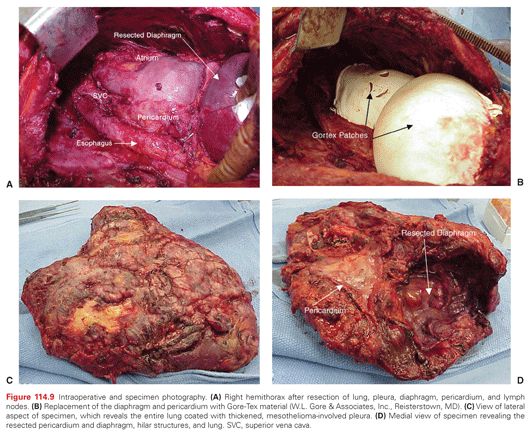
Extended P/D: parietal and visceral pleurectomy to remove all gross tumor with resection of the diaphragm and/or pericardium.
P/D: parietal and visceral pleurectomy to remove all gross tumor without diaphragm or pericardial resection.
Partial pleurectomy: partial removal of parietal and/or visceral pleura for diagnostic or palliative purposes but leaving gross tumor behind.
Palliation or MCR?: Only approximately 20% of patients presenting with pleural MPM will be candidates for an MCR. The decision to actually palliate a patient with mesothelioma begins with palliation of fluid in order to improve dyspnea or cough, as described previously. Patients who have an excellent result from drainage, with partial or full lung expansion, may then be considered candidates for MCR depending on other factors including (1) the experience of the thoracic surgeon, (2) the cardiopulmonary/functional status of the patient, and (3) patient/tumor demographics at presentation. Any preoperative staging radiologic examination that points to disease outside the involved hemithorax eliminates the use of an MCR. Thoracic surgeons at major centers with mesothelioma programs decide not only whether the patient is a candidate for cytoreduction or palliation but also the appropriate cytoreductive procedure. Patients who present with Eastern Cooperative Oncology Group performance status ≥2, or who have limiting cardiac reserve either with nonreversible myocardial ischemia or compromised ventricular function (ejection fraction <50%) are usually not candidates for cytoreductive procedures. Moreover, patients with compromised pulmonary function testing who are not felt to be candidates for pneumonectomy must be carefully evaluated for either extended P/D in that the cytoreduction should not compromise existing lung function but may recruit trapped lung and possibly improve dyspnea.
CONTROVERSY REGARDING PERFORMANCE OF EXTRAPLEURAL PNEUMONECTOMY
Extrapleural Pneumonectomy Results
The use of EPP for MCR has evolved since the first description alluded to previously. As detailed in a systematic review of the use of EPP for MPM,164 which included survival and perioperative outcomes, the median overall survival varied from 9.4 to 27.5 months, and 1-, 2-, and 5-year survival rates ranged from 36% to 83%, 5% to 59%, and 0% to 24%, respectively. Overall perioperative mortality rates ranged from 0% to 11.8%, and the perioperative morbidity rates ranged from 22% to 82%. In Sugarbaker and Wolf’s165 series, the mortality of EPP is reported at 3.4%, which has been achieved by improving preoperative staging, anesthesia, resection, reconstruction, and perioperative management of procedure-related complications. The chief complication is reversible atrial fibrillation, which occurs in 44.2% of patients and other major causes of morbidity are myocardial infarction (1.5%), epicarditis (2.7%), pulmonary complications (7.9%), thrombosis (6.4%), empyema (2.4%), technical complications (6.1%), and gastrointestinal complications (0.9%).166 Some of the technical issues include herniation of the heart due to failure of the pericardial patch or herniation of abdominal contents into the pleural space secondary to dehiscence of the diaphragmatic repair, vocal cord dysfunction secondary to recurrent laryngeal nerve injury, bronchopleural fistula, empyema, and prolonged respiratory failure. EPP alone does not appear to prolong survival. In most EPP series, median survival is <2 years, but 10% to 20% of patients are 5-year survivors. Patients with sarcomatoid histology and/or lymph node involvement have an even poorer prognosis and should not be considered as candidates for EPP.167
Trimodality Therapy in the Context of an Extrapleural Pneumonectomy
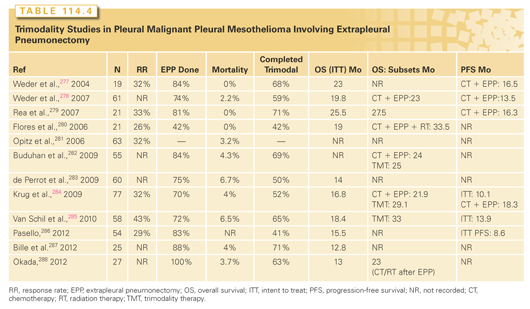
Trimodality therapy (TMT) for MPM began with evidence of a decreasing mortality and standardization of techniques for EPP, as well as the demonstration of increased tumor responses and survival with cisplatinum doublets using either gemcitabine168 or pemetrexed.169 TMT consists of induction chemotherapy, followed by EPP, and RT either as hemithorax radiation to the pneumonectomy cavity or as intensity modulated RT.170 Because of the morbidity and mortality with EPP, patient selection is a critical factor. The ideal patient is younger than 65 years old, has pure epithelial histology, an absence of lymph node involvement, and is physiologically able to tolerate pneumonectomy. Although EPP has been reported thus far as the primary surgical MCR technique in this selected group of patients, it should not be considered the only option, as detailed later. Table 114.4 describes the major TMT trials to date using EPP for MCR, and overall demonstrates a 3.1% mortality for EPP, along with a 27.7-month median survival in the 60% of presenting patients who completed the full package.
Randomized Trials of Surgery for Malignant Pleural Mesothelioma Involving Extrapleural Pneumonectomy
As of 2002, only one randomized trial involving surgery for MPM had been performed,171 and that trial did not address the issue of the utility of surgery, specifically EPP in MPM. In an attempt to define the efficacy of EPP, Mesothelioma and Radical Surgery (MARS) was originally designed as a randomized trial with 670 patients needed comparing induction chemotherapy, EPP, and postoperative RT to induction therapy but no EPP.172 When difficulty arose in recruiting patients, an initial feasibility study was performed randomizing 50 patients to EPP or no EPP. The final results of the trial demonstrated that it may be feasible to randomize MPM patients to a surgical or nonsurgical arm; however, the conclusions of the study’s authors have caused considerable controversy among surgeons and medical oncologists dealing with MPM. The study as published revealed nonsignificant differences in the 6, 12, 18 months and median survivals comparing the surgery arm to the nonsurgical arm, with a median survival of 14.4 months versus 19.5 months, respectively. Amongst many practitioners, the study has been interpreted not only as a condemnation of EPP in the management of MPM but also as an indictment of surgery as nonefficacious in the disease. One of the main criticisms of the study includes an 18% operative mortality from EPP, which is to be contrasted to the data noted previously for other phase 2 TMT trials (3.1%). Moreover, other flaws included (1) the lack of power of the study, which demanded a sample size of 670 randomized individuals; (2) the fact that the preoperative chemotherapy was not standardized; (3) the crossover of individuals from one arm to the other in six instances; (4) the lack of data on the interval from the end of chemotherapy to surgery; and (5) only one-third of the individuals received the full TMT while, as demonstrated previously, 60% of the individuals in the phase 2 setting completed the TMT and recorded a median overall survival of 27.7 months.173
EVOLUTION OF LUNG SPARING APPROACHES FOR MACROSCOPIC COMPLETE RESECTION
Questions regarding the ability to perform the same degree of MCR and also spare lung parenchyma arose while studies investigating TMT with EPP as well the MARS trial were accruing patients. A retrospective review of 663 consecutive patients (538 men and 125 women with MPM who underwent EPP or P/D at three US mesothelioma centers) reported an operative mortality of 7% for EPP (n = 27/385) and 4% for P/D (n = 13/278).174 The cytoreduction for these patients was described as a macroscopic tumor clearance, both pleurae, and as needed resection of the diaphragm and/or pericardium. Besides significant survival advantages seen for earlier stage (p <0.001), epithelioid versus nonepithelioid histology (p <0.001), multimodality therapy versus surgery alone (p <0.001), and female versus male gender (p <0.001), the type of operation, EPP versus P/D (p <0.001), also influenced survival. Multivariate analysis demonstrated a 1.4-fold greater chance for death with EPP (p
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree