5 Behavior and Dietary Modification in the Prevention of Colon Cancer
Introduction
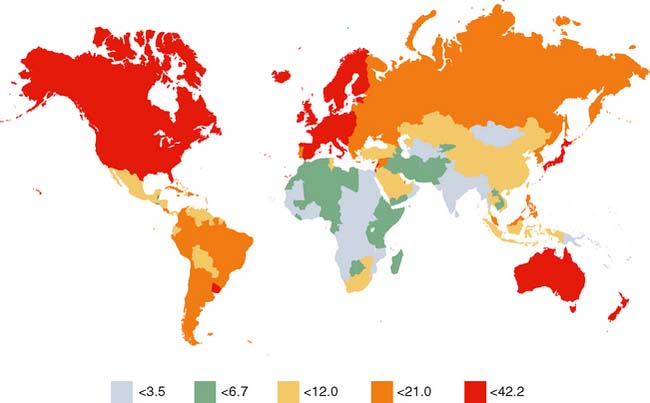
Colorectal cancer is found throughout the world (Fig. 5-1). In the United States, it is the second leading cause of cancer. In addition to genetics, environment and lifestyle play important roles in the development of colorectal cancer. The effects of environmental influences on human cancer became evident when early epidemiologic studies showed that cancer rates vary strikingly among countries—by five- to tenfold—and that immigrants moving from low-risk to high-risk countries adopt the rates of the new country.1,2 Moreover, Burkitt’s hypothesis in the 1970s that colon cancer rates of European lineage populations in South Africa were much higher than those of the native Bantu populations—theoretically because of an increased intake of refined cereals, protein, fat, and sugar—added further interest in diet as a possible etiologic factor of malignancy.3 By comparing rates of cancer mortality around the world, Doll and Peto4 in 1981 “guesstimated” that approximately 90% of colon cancers in the United States could be attributed to environmental factors and therefore could have been avoided. Although this theoretical maximum was an obvious overestimate, the analysis provided an important starting point for subsequent studies on environmental causes of cancer and strategies for cancer prevention.
Beginning in the 1980s, randomized controlled trials of nutritional supplementation were used in an effort to isolate individual components of foods that could be responsible for the effect of diet on colon cancer. However, intervention trials have their own challenges as well, because the timing of intervention, duration of the trial, and compliance of the subjects can greatly influence the results.
In this chapter, we review the scientific evidence on dietary and lifestyle factors that are related to primary prevention of colorectal cancer. Chemoprevention of colorectal cancer is discussed in Chapter 6.
Macronutrients
Red Meat: Intake and Cooking
Reports from the World Cancer Research Fund, the Chief Medical Officer’s Committee on Medical Aspects of Food, and a World Health Organization consensus statement all reached similar conclusions regarding a possible increased risk of colorectal cancer associated with high intake of red meat.5–7 The bulk of evidence supports this view, but the data are not entirely consistent. Epidemiologic studies show conflicting results, with countries such as Greece having an increasing meat intake and low colorectal cancer rates and with Australia and the United Kingdom having decreasing meat intake but increasing colorectal cancer rates.8 Most case-control studies, however, have shown a positive association between red meat intake and colon cancer, with a few exceptions.9–14 Cohort studies have also shown a statistically significant association between processed meat and risk of colorectal cancer.15–17 Other cohort studies have not confirmed this finding, however.18 The Nurses’ Health Study, which is one of the largest and longest-running studies on factors affecting women’s health and which comprises a cohort of about 90,000 nurses in the United States, identified consumption of red meat as a colon cancer risk factor.19 However, in the Polyp Prevention Trial (PPT), a randomized controlled trial that included 1905 subjects followed up for approximately 4 years, the adoption of a diet that de-emphasized red meat did not decrease the recurrence of colorectal adenomas.20 It has been suggested that poor adherence to the diet and the short duration of the trial might be reasons that this study did not support the findings of observational studies.
A meta-analysis of 34 case-control studies and 14 cohort studies conducted in 2002 concluded that high intake of red meat, and particularly processed meat, was associated with a moderate but significant increase in colorectal cancer risk.21 Average relative risk (RR) and 95% confidence interval (CI) for the highest quantile of consumption of red meat were 1.35 (CI 1.21–1.51) and of processed meat 1.31 (CI 1.13–1.51). A recent meta-analysis of prospective studies published through March 2006, which included 15 prospective studies on red meat (involving 7367 cases) and 14 prospective studies on processed meat consumption (7903 cases), showed that consumption of red meat and processed meat was positively associated with an increased risk of both colon and rectal cancer, although the association with red meat appeared to be stronger for rectal cancer.22 The estimated summary relative risks were 1.28 (95% CI 1.18–1.39) for an increase of 120 g/day of red meat and 1.09 (95% CI 1.05–1.13) for an increase of 30 g/day of processed meat. Specifically, the relative risk for colon cancer was 1.21 (1.05–1.40) and for rectal cancer 1.56 (1.25–1.95). The evidence that prolonged high consumption of red and processed meat may increase the risk of cancer in the distal portion of the large intestine as opposed to the proximal colon has been somewhat consistent.15,16,22
The mechanism by which high red or processed meat intake might increase the risk of colorectal cancer is entirely unclear. Some experimental data suggest that protein escapes digestion in the small intestine and is degraded by the colonic microflora, giving rise to a number of products that may promote colorectal cancer (ammonia, phenols, indoles, amines). Processed meat may also contribute to high colonic nitrosamine concentrations or may be a source of heme iron, which can act as a peroxidant. Recent studies have focused on the role of cooking methods of meat in the increased risk. Heterocyclic amines (HCAs), which are formed on the surface of heavily cooked meats exposed to high temperatures, are potent mutagens. Some studies have reported that increased consumption of well-done or fried red meat with a heavily browned surface is associated with an increased risk of colorectal cancer, but the risk is not increased among those who consume meat with a medium or lightly browned surface.9,23,24 Not all studies have confirmed this finding, however.25,26 It has also been suggested that acetylation polymorphisms can influence the risk of colorectal neoplasia and that subjects with a fast acetylator status (i.e., increased activity of the N-acetyltransferase enzymes), who would readily activate aromatic and heterocyclic amine carcinogens within the colon to their ultimate carcinogenic forms, are especially sensitive to brown meat and exhibit a greater colorectal cancer risk.27
These findings have not been replicated by more recent studies.28 Future investigations should further determine the role of heterocyclic amines in colorectal carcinogenesis and whether genetic polymorphisms influence susceptibility to their adverse effects. The role of chemicals derived from cooked meat in colorectal neoplasia has not yet been clearly defined.
Dietary Fat
Data from earlier epidemiologic studies had generally shown an association between risk of colon cancer and fat intake.29–31 Rates of colorectal cancer in various countries were strongly associated with per-capita consumption of animal fat.1,32 The incidence of colorectal cancer in Japan began rising steeply after World War II, and this coincided with a 2.5-fold increase in fat intake.33 These data led to the hypothesis that dietary fat increases excretion of bile acids, which can be converted to carcinogens. The combined epidemiologic and experimental evidence led the Committee on Diet, Nutrition, and Cancer of the National Research Council in 1986 to recommend a reduction in dietary fat from 40% to 30% of calories as a possible means of reducing cancer risk.34 However, more recent studies have suggested that this is not supported by strong evidence and that the association seen between colorectal cancer and animal fat might be explained by factors in red meat other than its fat content. A meta-analysis of data from 13 case-control studies conducted in populations with differing colorectal cancer rates and dietary practices found a significant association between total energy intake and colon cancer but no association between dietary fat intake and risk of colorectal cancer independent of total energy.35 The Nurses’ Health Study, one of the best cohort studies of diet and colon cancer, showed a positive association between animal fat intake and the risk of colon cancer.19 The relative risk for the women in the highest quintile compared with that in the lowest quintile was 1.89 (95% CI 1.13–3.15). Nevertheless, a subsequent multivariate analysis of these data, which included red meat and animal fat intakes in the same model, showed no association with animal fat, whereas red meat intake remained significantly predictive of colon cancer.36 Similarly, other large cohort studies from the United States and the Netherlands found no direct relation between fat intake and mortality due to colon cancer.17,37,38 Four randomized controlled trials that investigated the usefulness of low-fat diets in colorectal adenoma and cancer prevention failed to show protection.20,39–41 As previously mentioned, results of the Polyp Prevention Trial (PPT) showed no reduction in the risk of colorectal adenoma recurrence over approximately 4 years.20 Similarly, in the Women’s Health Initiative (WHI), a large randomized controlled trial involving 36,282 postmenopausal women followed up for 7 years, a low-fat dietary pattern did not affect colorectal cancer risk.41 Potential reasons for the null results in these trials may reflect the complex multistage process of colorectal carcinogenesis, timing of dietary change, duration of the study, as well as poor dietary adherence among participants in these trials. It is interesting that a recent subset analysis of the PPT trial identified a group of the most adherent subjects to a low-fat, high-fiber, high-fruit and high-vegetable diet, defined as “super compliers” (n = 210). The authors observed 35% reduced odds of adenoma recurrence and nearly 50% lower odds of multiple and advanced adenoma recurrence among super compliers compared with controls, suggesting that consistent adherence to a low-fat, high-fiber, high-fruit and high-vegetable diet may be effective in preventing recurrence of colorectal adenomas and possibly in preventing colorectal cancer.42 Furthermore, although increased total fat and animal fat intake may increase the risk of colorectal carcinogenesis, increased consumption of polyunsaturated fats such as fatty acids found in fish oils may conversely decrease the risk of colorectal cancer.7,43 Ongoing and future investigations are warranted to determine the exact relation between fat and the risk of colon cancer.
Fiber
Increased dietary fiber has long been postulated as a protective factor against the development of colorectal cancers (Box 5-1). However, a consensus has yet to be reached owing to wide differences in study results over the past 30 years.
Box 5-1 Examples of High-Fiber Foods
Adapted from Mayo Clinic E-Newsletter, Available at www.mayoclinic.com/health/high-fiber-foods/NU00582.
Strong evidence from both animal and human studies has shown that consumption of a diet high in fiber can reduce the risk of colorectal cancers by several mechanisms, including increase in stool bulk and decrease in stool transit time, increased binding of potential mutagens, fermentation of fiber into short-chain fatty acids (SCFA), and reduction of luminal pH. An increase in dietary fiber and a subsequent increase in stool bulk were the first of these factors shown to be inversely linked to the risk of colorectal cancers and were thought to act by reducing transit time and thus the amount of time that the colonic mucosa is exposed to other diet carcinogens.44,45 This increase in bulk may also provide a chemical or physical barrier for the colonic mucosa to known carcinogens in the diet, such as high concentrations of the secondary bile salts lithocholic and deoxycholic acids.46,47 In addition, some fiber types are fermented by large bowel bacteria into short-chain fatty acids, particularly butyrate, which has been shown to inhibit the growth of hyperproliferative epithelium in the case of ulcerative colitis and bile salt exposure.48–52 Furthermore, butyrate has been shown to inhibit histone deacetylase, causing histone hyperacetylation and thus disinhibition of gene expression and leading to cell apoptosis and unresponsiveness to local growth factors.48,53,54 This colonic fermentation further reduces colonic pH, which has been shown to reduce secondary bile salt solubility and is associated with lower cancer risk in humans.46,47,55 Furthermore, reduction in colonic pH has been shown to shift gut flora away from a more anaerobic mix that is associated with an increased risk of colorectal cancer. This action is thought to be through the production of carcinogens such as heterocyclic amines and phenolic compounds by anaerobic bacteria of Clostridia and Bacteroides species.46,55
Investigations of large international, populationbased data, such as one by Bingham and associates,61 have shown a strong inverse correlation between fiber consumption and colorectal cancer; furthermore, most case-control studies have shown that increased dietary fiber has a protective effect against the development of colorectal cancers.1,11,56–71 However, a few case-control studies have failed to show this protective effect; moreover, all of these case-control and population-based studies are plagued by confounding factors such as inconsistent estimation of portion sizes and sources of fiber in the diet.72–75
To help elucidate the impact of these seemingly contrary results, Howe and associates35 pooled the data from 13 case-control studies regarding the influence of dietary fiber on colorectal cancer development and found that, in aggregate, the relative risk of cancer in the highest quintile of fiber consumption compared with that in the lowest was 0.53 (95% CI 0.47–0.61), a figure similar to other large studies and pooled results. However, these results were highly criticized because they did not take into account the quality of individual studies. Although Howe’s study35 showed serious methodologic concerns, the study of Friedenreich and associates76 showed that using a random-effects or fixed-effects model to account for differences in the quality of the same 13 studies did not drastically change the odds ratio for the interquintile range (random effects: odds ratio [OR] 0.46, 95% CI 0.34–0.64; fixed-effects: OR 0.51, 95% CI 0.44–0.59), indicating that the variability in case-control results could not be attributed to quality of study design.
Buoyed by these results, several interventional trials have been undertaken to elucidate the effect of dietary fiber on subsequent colorectal polyp development. However, these were generally of very short duration (2 to 4 years), examined only high-risk populations (personal history of polyps or cancer, familial adenomatous polyposis), and, because of a short follow-up, looked only at polyp development, not cancer development. Consequently, there has been little to no indication that the supplementation of fiber over a short period of time reduces the risk of polyp development in these high-risk populations.20,39,40,77–81 There is one exception to this trend: Alberts and associates82 showed in a randomized, double-blind, placebo-controlled trial that wheat bran fiber significantly reduced fecal bile acid content.
Vegetables and Fruits
Adding some confusion to the fiber debate is the inclusion of dietary fiber from vegetables and fruits, which may have additional dietary micronutrients that also may influence the development of colorectal cancer. For instance, Negri and associates65 have shown that fiber intake from a vegetable source had the greatest reduction of associated colorectal cancer risk in Italian patients. The majority of studies have shown a reduction of colorectal cancer risk with the consumption of vegetables, fruits, or both.57,65–67,69,71,73,83–87 Of note, some studies have shown no association for all patients or for selected populations, such as cigarette smokers.17,88,89
Of particular interest is consumption of members of the Allium genus of plants such as garlic, onions, shallots, chives, and leeks. Compounds found in these vegetables, such as diallyl sulphide, flavonols quercetin, and kaempferol and glutathione, have been implicated as anticarcinogens.90 Consumption of Allium vegetables have been shown to be associated with a decreased risk of colorectal cancer, particularly distal cancer in 9 of the 16 published studies on the topic, including the only two randomized controlled trials in the group.17,31,66,67,71,84,85,89–96 Furthermore, a meta-analysis by Fleischauer and associates,97 including seven of these studies, and a review of the quality of literature by Ngo and colleagues98 both showed a preponderance of quality evidence in favor of an inverse association between Allium genus plant consumption and colorectal cancer.
Insulin and Glycemic Load
Risk factors for colorectal cancer and insulin resistance syndromes show considerable overlap, such as central obesity and low physical activity (discussed elsewhere in this chapter), leading some experts to postulate that increased insulin levels promote colorectal cancers.99–101 For instance, several researchers have shown that non–insulin-dependent diabetes mellitus is an independent risk factor for colorectal cancer even when controlling for their joint risk factors.102–104 This was clearest in the Iowa Women’s Health Study, which showed a link between those with recently diagnosed type 2 diabetes but not for those with a longstanding diabetes diagnosis, the latter of which would presumably not be associated with elevated insulin levels.38
In addition, high consumption of foods that cause a dramatic spike in postprandial insulin, so-called high glycemic load foods such as sugar or sugar-dense foods, has been shown to correlate with the development of colorectal cancer in several population-based and case-control studies.38,83,86,105–112 Notably, several other studies have shown no significant risk increase for those with high sugar consumption but have shown a correlation between high overall energy intake and colorectal cancers.1,10,14,69,84,85,113–117 Furthermore, Ma and associates118 showed with prospective collected data that, even in nondiabetic men, circulating levels of plasma C-peptide correlated with the development of colorectal cancer (risk ratio for the highest quintile to the lowest = 3.4, P = .02), a finding previously shown in elderly men and in women.104,119
Coffee and Tea
Several studies have examined the impact of coffee and tea drinking on the development of colorectal cancers. Coffee drinking has been shown to be associated with a lower risk of colorectal cancer in several studies,10,14,91,106,113,120–124 but several other studies showed no association.125–131 One review article has shown a protective effect of coffee consumption against the development of colorectal cancers.132 In contrast to the consumption of black teas, which seems to have no association with the development of colorectal cancers in several studies,133–135 green teas have been shown to have anticancer effects, primarily through a compound called epigallocatechin gallate.136 Furthermore, green teas have been shown to be associated with reduced colorectal cancer risk, including in a review paper by Bushman.137–140
Dairy Foods and Probiotics
It is not surprising that attempts to compare dairy products with the risk of colorectal cancer have resulted in extremely varied results owing to the fact that dairy foods tend to contain high levels of both dietary fat and calcium (discussed later in this chapter). Most studies have shown that the consumption of dairy foods has a nonsignificant protective effect on the development of colorectal cancer.38,73,130,141,142 However, the consumption of milk products significantly reduced the risk of colorectal cancers in two pooled datasets.143,144 Furthermore, in one study that has examined skim and whole milks separately, it was found that skim milk consumption was associated with reduced colorectal cancer risk but whole milk consumption was not.145 Finally, consumption of milk was shown to be protective against colorectal cancer in male smokers in Finland.146
In addition, the impact of fermented milk products (yogurt, buttermilk, and cheeses) and unpasteurized milk has been investigated in association with colorectal cancers. For the former, there is some evidence that lactic acid bacteria such as Lactobacillus and Bifidobacterium can reduce cell proliferation, urinary mutagenicity, and bacterial enzyme activity that can produce carcinogens.147,148 Although no evidence has been seen from cohort studies of protective benefits from fermented milk products, three of the larger case-control studies have shown a protective benefit even when controlling for other factors.149–153 It has been hypothesized that unpasteurized milk, in contrast, increases colorectal cancer risk through exposure to bovine pathogens, particularly bovine leukemia virus.154,155 However, using data from the Iowa Women’s Health Study, Sellers and colleagues156 actually found a protective benefit in the consumption of unpasteurized milk with regard to the development of colorectal cancer, which became nonsignificant after controlling for other factors.
Vitamins and Micronutrients
Calcium
A possible protective effect of calcium on colorectal cancer was suggested primarily from results of in vitro animal studies. It has been hypothesized that dietary calcium provides protection from colon cancer by binding with fat and reducing the concentrations of free fatty acids and bile in the lumen of the bowel, thus reducing their proliferative influence on the colon mucosa.157 It has also been proposed that calcium can directly reduce mucosal proliferation.158 The epidemiologic evidence, however, has been inconsistent. Several, but not all, studies showed a weak inverse association of calcium intake with risk of colorectal adenoma or cancer.159 Pooled results from two large prospective cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), with a follow-up of at least 10 years, showed an inverse association between calcium intake and distal but not proximal colon cancer. Furthermore, the authors showed that the benefit of calcium intake has a threshold effect, in that intake beyond approximately 700 mg/day appeared to have minimal additional effect, suggesting that calcium intake beyond moderate levels may not be associated with a further risk reduction.160 Another cohort of 35,216 women from Iowa showed that calcium intake was associated with a significantly reduced risk of colon cancer (RR 0.5, CI 0.3–0.7) among women with a negative family history but not among women with a positive family history.161
Several randomized controlled trials have tested the protective role of calcium supplementation against adenoma recurrence and colorectal cancer.80,162–165 The trial reported by Baron and colleagues162 found that calcium supplementation at 1200 mg/day for 4 years reduced adenoma recurrence by 15%. This effect was shown to extend as long as 5 years after cessation of supplementation.166 Bonithon-Kopp and colleagues80 reported a 34% reduction in the recurrence rate of adenomas after taking 2000 mg calcium per day for 3 years, but the result did not reach statistical significance. In the WHI, the latest and largest placebocontrolled randomized trial, which involved 36,282 postmenopausal women, daily supplementation with a combination of 1000 mg calcium and 400 units of vitamin D for 7 years had no effect on the incidence of colorectal cancer.41 However, one of the major criticisms of this study was that the mean baseline calcium intake in these women was 1151 mg/day, resulting in a total calcium intake of approximately 2150 mg/day, levels that, based on the prospective cohort study data, are consistent with no effect. As a result, the WHI did not test whether calcium supplementation would convey protection among individuals with low or moderately low baseline intakes of this nutrient.167
On the whole, the literature supports a moderate protective effect of calcium against colorectal cancer. A recent summary of systematic reviews from the World Cancer Research Fund concluded that there is a modest, significant inverse association between calcium intake and colorectal cancer.168 The latest American Cancer Society Guidelines on Nutrition and Cancer Prevention also recommend 1000 mg calcium per day for adults up to age 50 years and 1200 mg/day for those older than 50 years.169
Vitamin D
Vitamin D was proposed as a potential protective factor against colon cancer in the 1980s, when correlational studies revealed that colon cancer mortality rates in the United States were highest in places where populations were exposed to the least amounts of natural light, including major cities and rural areas in high latitudes.170 Vitamin D is a fat-soluble vitamin that can be obtained from fatty fish, fish oil, and fortified dairy products, or it can be synthesized endogenously in the skin after exposure to ultraviolet light. Its primary role is in the regulation of calcium homeostasis in the body. Putative biologic activities related to the presumable protective effects of vitamin D in colon neoplasia include its pro-differentiating and antiproliferative effects through induction of cell-cycle arrest and apoptosis.171–173 Four prospective cohort studies have reported inverse associations between dietary vitamin D and colorectal cancer, but this was significant only in the Western Electric Study, a study of men only.174 In the Iowa Women’s Health Study and the Health Professionals Follow-up Study, a significant inverse relation was observed for vitamin D intake and risk of colon cancer, although this was attenuated and was no longer significant after multivariate adjustment.175,176 Secondary analysis of the data from the prospective Nurses’ Health cohort study showed an inverse association between intake of vitamin D and risk of colorectal cancer.177 Some case-control studies have shown a decreased risk of colorectal cancer with higher levels of dietary vitamin D, whereas others have not.83,152,178,179 However, a recently published meta-analysis of 17 epidemiologic studies demonstrated an inverse association between vitamin D and colorectal adenoma incidence and recurrence. The highest quintile of vitamin D intake was associated with an 11% decreased risk of colorectal adenomas compared with low vitamin D intake (OR 0.89, 95% CI 0.78–1.02).180 Moreover, further analysis of the data from the randomized trial of Baron and colleagues184 showed that higher serum 25-(OH) vitamin D levels were associated with a reduced risk of adenoma recurrence among subjects receiving calcium supplements. This suggests that calcium supplementation and vitamin D status act largely synergistically to reduce the risk of colorectal adenoma recurrence.
The largest randomized trial of combined calcium and vitamin D is the WHI.41 In this trial, 18,716 women were randomized to receive both 1000 mg/day of elemental calcium and 400IU/day of vitamin D while 18,106 women received matching placebo. After a mean follow-up of 7 years, the combination of calcium and vitamin D had no effect on the incidence of colorectal cancer. This study had several potential limitations. Only a single regimen was evaluated, and it is therefore unknown whether other formulations would have changed the results. The calcium as well as vitamin D doses that were used might have been insufficient to demonstrate a protective effect, particularly given the percentage of participants who were not fully compliant throughout the study. A larger dose of vitamin D than that used in the WHI trial may be necessary for any measurable chemopreventive effect. Therefore, the relation among vitamin D status, dose of vitamin D supplements, and colon cancer incidence or recurrence still remains unknown.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree