INTRODUCTION
SUMMARY
Basophils and mast cells share biochemical and functional characteristics, but they are not identical.* In humans, basophils are the least frequent of the three granulocytes, typically accounting for less than 0.5 percent of blood leukocytes. Basophils circulate as mature cells and can be recruited into tissues, particularly at sites of immunologic or inflammatory responses, but they ordinarily do not reside in tissues. By contrast, mast cells typically are derived from blood precursors that lack many of the characteristic features of the mature cells and complete their maturation in the tissues. The mature mast cells can reside in tissues for long periods of time. Mast cells are particularly abundant near blood vessels and nerves and in connective tissues beneath surfaces that are exposed to the external environment, such as the skin, gastrointestinal and urogenital tracts, and respiratory system. Tissue mast cell numbers can increase at sites of parasite infection or in association with certain chronic allergic diseases or other forms of pathology, by recruitment and local maturation of blood precursors and by proliferation of resident mast cells.
Mast cells and basophils express the high-affinity receptor for immunoglobulin (Ig) E (FcεRI) on their surface. Both cell types can be triggered to release potent mediators in response to activation via FcεRI, for example, when their cell-bound IgE recognizes bivalent or multivalent allergens. Accordingly, mast cells and basophils have long been regarded as important effector cells in asthma, hay fever, and other allergic disorders. The cells’ cytoplasmic granule-associated preformed mediators, including histamine and certain proteases, their lipid mediators (such as prostaglandin D2 and leukotriene C4), which are generated upon activation of the cells, and their cytokines, growth factors, and chemokines contribute to many of the characteristic signs and symptoms of these diseases. However, several lines of evidence indicate mast cells and basophils also contribute to protective host responses associated with IgE production, especially those directed against parasites. In mice, mast cells can enhance innate and acquired (IgE-dependent) defense against animal venoms and also can contribute to host defense in innate immune responses to certain bacterial infections. Mast cells and basophils also may express positive and negative immunoregulatory functions through cytokine production and other mechanisms.
Although a variety of systemic disorders are associated with changes in the numbers of blood basophils and many pathologic processes can be associated with changes in the numbers of tissue mast cells, patients with primary deficiencies in basophils appear to be exceedingly rare (if they exist at all). Patients with a primary deficiency of tissue mast cells have not been reported. By contrast, neoplastic processes can affect both of the lineages. Increased numbers of basophils may be present in association with myeloproliferative neoplasms and several forms of myeloid leukemia. Increased numbers of basophils, sometimes to levels of 20 to 90 percent of blood leukocytes, occur in virtually all patients with chronic myelogenous leukemia. The basophils associated with both chronic myelogenous and acute myeloid leukemias are themselves part of the neoplastic clone. The management of patients with “basophilic leukemia” can be complicated by shock as a result of massive release of histamine and other mediators in association with acute cytolysis.
Disorders of mast cell hyperplasia/neoplasia include solitary mastocytomas, the pathogenesis of which is uncertain, the spectrum of disorders encompassed in the term mastocytosis, in which significantly increased numbers of mast cells occur in the skin and/or other organs, and mast cell leukemia. The most common form of mastocytosis, indolent systemic mastocytosis, typically presents with urticaria pigmentosa involving the skin, although other organs may be involved. Patients with indolent systemic mastocytosis have the best prognosis and can expect a normal life span. The prognosis of systemic mastocytosis with associated clonal, hematologic non–mast-cell-lineage disease depends on the course of the associated disease. Patients with aggressive systemic mastocytosis have a guarded prognosis because of complications arising from rapid increases in tissue mast cell numbers. Patients with mast cell leukemia, who often present with large numbers of immature mast cells in the blood at the time of diagnosis, have a fulminant and rapidly fatal course. Most adult patients with mastocytosis have gain-of-function mutations affecting KIT, which encodes the receptor for the major mast cell growth factor stem cell factor (also known as kit ligand and mast cell growth factor). Some pediatric patients with mastocytosis reportedly have the same Asp816Val gain-of-function KIT mutation observed in most adult patients. Some pediatric patients have a dominant inactivating KIT mutation, whereas others lack KIT mutations entirely.
Acronyms and Abbreviations:
AML, acute myeloid leukemia; ASM, aggressive systemic mastocytosis; CML, chronic myelogenous leukemia; CPA3, carboxypeptidase A3; DEXA, dual-energy x-ray absorptiometry; ET-1, endothelin-1; FcεRI, high-affinity receptor for IgE; gp120, glycoprotein 120; H&E, hematoxylin and eosin; HLA, human leukocyte antigen; IFN-α, interferon α; Ig, immunoglobulin; IL, interleukin; ISM, indolent systemic mastocytosis; MCAS, mast cell activation syndrome; MCL, mast cell leukemia; MCP, mast cell–committed progenitor; MCS, mast cell sarcoma; MMAS, monoclonal mast cell activation syndrome; mMCP, mouse mast cell protease; PUVA, psoralen ultraviolet A; qPCR, quantitative polymerase chain reaction; SCF, stem cell factor; SCT, stem cell transplantation; SM-AHNMD, systemic mastocytosis with associated clonal hematologic non–mast-cell-lineage disease; Th, T-helper; TLR, toll-like receptor; TNF-α, tumor necrosis factor α; UP, urticaria pigmentosa; VEGF, vascular endothelial growth factor; VIP, vasoactive intestinal polypeptide.
*Acknowledgment: This work was in part supported by the Division of Intramural Research, NIAID.
DISTINGUISHING FEATURES OF BASOPHILS AND MAST CELLS
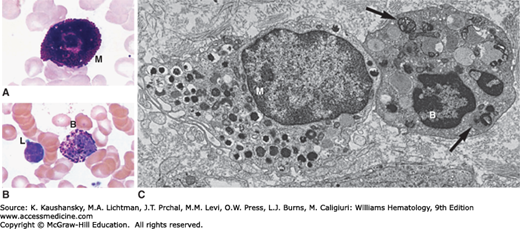
Despite certain similarities in biochemistry and function, mammalian basophils and mast cells are not identical (Fig. 63–1).1,2,3,4,5 The distinction was appreciated by Paul Ehrlich, who described the histochemical staining characteristics of these cells in the late 19th century. Much evidence indicates that basophils share a common precursor with other granulocytes and monocytes.1,2,3,4,5 Basophils have a short life span5,6 and retain granulocytic features even after emigrating into tissues (Fig. 63–1C).7
Figure 63–1.
A. Mast cell (M) in a Wright-Giemsa–stained marrow aspirate. B. A basophil (B) and lymphocyte (L) in a Wright-Giemsa-stained film prepared from the buffy coat of blood from a normal donor. C. Transmission electron micrograph illustrating a mast cell (M) and basophil (B) in the ileal submucosa of a patient with Crohn disease. The mast cell is a larger, mononuclear cell with a more complex plasma membrane surface and cytoplasmic granules that are smaller and more numerous than those of the basophil. In this section plane, the basophil exhibits two nuclear lobes. Several basophil cytoplasmic granules contain whorls of membranes (arrows). Osmium collidine uranyl en bloc processing. (C, reproduced with permission from Dvorak AM, Monahan RA, Osage JE, et al: Crohn’s disease: Transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol 11(6):606–619, 1980.)
The basophil is the least-common granulocyte in human blood, with a prevalence of approximately 0.5 to 0.6 percent of total leukocytes and approximately 0.3 percent of nucleated marrow cells.8,9 The basophil’s prominent metachromatic cytoplasmic granules allow unmistakable identification in Wright-Giemsa–stained films of blood (see Fig. 63–1B) or marrow, but accurate enumeration requires absolute counting methods.9,10 Differential counts of blood films yield valid results only if the percentage of basophils is substantially elevated or many thousands of leukocytes are counted.
Interleukin (IL)-3 promotes the production and survival of human basophils in vitro3,11 and induces basophilia in vivo.12 Findings in IL-3 –/– mice indicate IL-3 is not necessary for the development of normal numbers of marrow or blood basophils, but is important for the marrow and blood basophilia associated with certain T-helper (Th) 2 cell-associated immunologic responses.13,14 Basophils also express receptors for several other cytokines (Table 63–1).5,15 IL-3 and many of these other cytokines, including IL-33, can modulate basophil function, for example, by inducing mediator release directly and/or by augmenting the cells’ ability to release mediators in response to immunoglobulin (Ig) E-dependent challenge.3,5,12
| Characteristics | Basophils | Mast Cells |
|---|---|---|
| NATURAL HISTORY | ||
| Origin of precursor cells | Marrow | Marrow |
| Site of maturation | Marrow | Connective tissue (a few in marrow) |
| Mature cells in circulation | Yes (usually <1% of blood leukocytes) | No |
| Mature cells recruited into tissues from circulation | Yes (during immunologic, inflammatory responses) | No |
| Mature cells normally residing in connective tissues | No (not detectable by microscopy) | Yes |
| Proliferative ability of morphologically mature cells | None reported | Yes (limited; under certain circumstances) |
| Life span | Days (like other granulocytes) | Weeks to months (according to studies in rodents) |
| Major growth factor | IL-3 | SCF |
| MEDIATORS | ||
| Major mediators stored preformed in cytoplasmic granules | Histamine, chondroitin sulfates, tryptase,* chymase,* carboxypeptidase A,* neutral protease with bradykinin-generating activity, β-glucuronidase, elastase, cathepsin G-like enzyme, major basic protein, Charcot-Leyden crystal protein | Histamine, heparin,* chondroitin sulfates,* chymase,* tryptase,* cathepsin G,* carboxypeptidases, major basic protein, acid hydrolases, peroxidase, phospholipases |
| Major lipid mediators produced on appropriate activation | Leukotriene C4 | Leukotriene B4, prostaglandin D2, leukotriene C4, platelet-activating factor |
| Cytokines released on appropriate activation | IL-4, IL-6, IL-13, GM-CSF, VEGF-A, leptin | TNF, TGF-β, IFN-α, VEGF-A–D, IL-6, IL-11, IL-13, IL-16, IL-18, GM-CSF, NGF, PDGF (mouse and human mast cells can secrete many more; see text) |
| Chemokines | IL-8 (CXCL-8), MIP-1α (CCL3), Eotaxin (CCL-11), MIP-5 (CCL15) | IL-8 (CXCL-8), I-309 (CCL-1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL-4), MCP-3 (CCL-7), RANTES (CCL-5), Eotaxin (CCL-11) |
| SURFACE STRUCTURES | ||
| Ig receptors | FcεRI, FcγRIIA, FcγRIIB | FcεRI, FcγRI (after IFNγ exposure), FcγRIIA |
| Cytokine or growth factor receptors for: | IL-1, IL-2 (CD25), IL-3, IL-4, IL-5, IL-6, IL-8, and IL-33; chemokines (CCR1, -2, -3, -5; CXCR1, -2, -4); and interferons; SCF (basophils express variable numbers of the SCF receptor, Kit) | SCF (ligand for Kit), IFN-γ, IL-4, IL-5, IL-6, IL-9, and IL-33; chemokines (CCR1, -3, -4, -5, -7; CXCR1, -2, -3, -4, -6); thrombopoietin receptor (CD110), GM-CSF, NGF |
| TLRs | TLR-2, -4 (but lack CD14) | TLR-1, -2, -3, -4, -5, -6, -7, -9 |
Mast cells normally reside in the connective tissue, particularly beneath epithelial surfaces and around blood vessels, and, in some species, in serous cavities.1,4,16,17 Mast cells are derived from hematopoietic precursors.16,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21 Except for a numerically minor population of mast cells residing in the marrow (see Fig. 63–1A),8 this lineage completes its program of maturation in other tissues.16,17,18,19,20,21 Unlike basophils, mast cells can be long-lived. At least some mast cells can proliferate in the tissues during a variety of inflammatory or reparative processes.1,16,17 Studies in mice, rats, nonhuman primates, and humans indicate many aspects of mast cell development are critically regulated by stem cell factor (SCF), the ligand for the KIT receptor tyrosine kinase.17,22,23,24 SCF is produced in membrane-associated and soluble forms, both of which are biologically active (Chap. 18).17,24 Beside promoting the migration, survival, proliferation, and maturation of cells in the mast cell lineage, SCF can directly promote mast cell mediator release23,25,26,27 and, at even lower concentrations, can augment mast cell mediator release in response to stimulation by IgE and antigen.25,26 Abnormalities affecting KIT are involved in the pathogenesis of certain types of mastocytosis (see “Disorders Affecting Mast Cells” below). Alterations in the production of SCF by fibroblasts and other cells may contribute to the changes in mast cell numbers that occur during many chronic inflammatory conditions and other pathologic responses.17,22,28
Variation in the morphologic, biochemical, and/or functional characteristics of mast cells from different anatomic locations or from the same organ or site has been reported in several mammalian species, including humans.16,17,21,29,30 This phenomenon, often referred to as mast cell heterogeneity, raises the possibility that mast cells of different phenotype express different functions in health or disease and may exhibit different sensitivities to pharmacologic manipulation. At least four mechanisms may account for phenotypic variation in mast cell populations: (1) factors promoting branching within the mast cell lineage; (2) factors influencing differentiation and maturation (within a single pathway or, if they occur, within multiple pathways); (3) factors modulating mast cell function; and (4) factors influencing local concentrations of exogenous substances not derived from mast cells but taken up and stored in mast cell granules. Of these four mechanisms, experimental evidence has been obtained for all but the first.21,30 Basophils can exhibit some variation in phenotypic characteristics also, such as immunoreactivity for tryptase, chymase, and carboxypeptidase A,15 or levels of expression of surface structures, including human leukocyte antigen (HLA)-DR, CD32 (FcγRII), and receptors for cytokines.5,31 Such variation in basophil mediator content and/or cell surface phenotype may reflect individual differences among different subjects and/or the effects of disease processes15 or the consequences of immunotherapy.31
Mature basophils and mast cells differ in morphology, natural history, tissue distribution, mediator production, cell-surface phenotype, growth factor requirements, and responses to drugs (see Fig. 63–1 and Table 63–1).1,2,3,4,5 Nevertheless, certain similarities of the two cells, taken together with evidence indicating that tissue mast cells are derived from circulating marrow-derived precursors,16,18 had suggested to some investigators that basophils might be circulating precursors of mast cells. However, the following evidence strongly favors the view that mature basophils represent terminally differentiated granulocytes and not circulating mast cell precursors: (1) no evidence has been presented, in any species, indicating that mature circulating basophils are capable of either mitosis or differentiation into mast cells; (2) rare reports of patients with hereditary or acquired abnormalities affecting basophil numbers or morphology indicate that eosinophils also may be affected in these disorders but not mast cells32,33,34; (3) morphologically identifiable human tissue mast cells can exhibit mitotic activity,35 indicating that mast cells can replicate independently of a stage resembling that of basophils, and (4) in mice, evidence indicates that a mast cell–committed progenitor (MCP) present in the marrow is developmentally distinct during hematopoiesis from a Sca-1lo granulocyte-macrophage progenitor.19,20 Evidence for a committed bipotential precursor of mast cells and basophils during hematopoiesis in the mouse has been reported by some studies36,37 but not others.19,20,38
Routine histologic methods are poorly suited for demonstrating basophils and mast cells. Optimal visualization is achieved in appropriately prepared 1 μm sections or by electron microscopy.1,2 By ultrastructure, human basophils are 5 to 7 μm in spherical diameter, exhibit a segmented or unsegmented nucleus with marked condensation of nuclear chromatin, and contain round or oval cytoplasmic granules surrounded by a membrane and containing a substructure of dense particles, less-dense matrix, and, in some granules, membrane whorls and Charcot-Leyden crystals (see Fig. 63–1C).1,2 A minor population of small, uniform granules is characteristically located near the nucleus.39 The cytoplasm of mature human basophils also contains glycogen particles, mitochondria, free ribosomes, and small membrane-bound vesicles. Lipid bodies are rarely present. Other organelles are inconspicuous.
In tissue sections, mast cells typically appear as either round or elongated cells, usually with a nonsegmented nucleus with moderate condensation of nuclear chromatin, and contain prominent cytoplasmic granules. Mast cell granules are smaller, more numerous, and generally more variable in appearance than in basophils and contain scroll-like structures, particles, and crystals, alone or in combination (see Fig. 63–1C).1,2 In contrast to the irregularly spaced blunt surface projections of basophils, mast cells are covered by uniformly distributed thin surface processes. Mast cells also differ from basophils in having many more cytoplasmic filaments and lacking cytoplasmic glycogen deposits. Human mast cells can contain numerous cytoplasmic lipid bodies.
The cytoplasmic granules of basophils and mast cells contain proteoglycans, consisting of sulfated glycosaminoglycans covalently linked to a protein core.40 These substances can be stained metachromatically with basic dyes (see Fig. 63–1A and B). In humans and murine species, individual mast cell populations can contain variable mixtures of heparin and chondroitin sulfate proteoglycans.29,40 Although the sulfated glycosaminoglycans of normal human blood basophils have not yet been characterized, two studies of the proteoglycans synthesized by blood leukocytes (containing 10 to 75 percent basophils) of five patients with myeloid leukemia indicate that such cells may produce solely chondroitin sulfates41 or a mixture of chondroitin sulfates (50 to 84 percent) and heparin (8 to 43 percent).42 Although the biologic functions of basophil and mast cell proteoglycans are not fully understood, in mice, heparin is required for normal packaging of certain neutral proteases in mast cell cytoplasmic granules.40,43 Both human mast cells and basophils synthesize and store histamine.1,4 Basophils represent the source of most (if not all) of the histamine present in normal human blood.44 Although macrophages,45 neutrophils,46 and platelets,47 as well as basophils,44 can produce histamine, mast cells represent the source of virtually all the histamine stored in normal tissues in mice, with the notable exceptions of the glandular stomach and parts of the central nervous system.48
In addition to proteoglycans and histamine, basophils and mast cells generate many other products that can influence the course of inflammatory processes (see Table 63–1).1,3,4,5,49,50,51 These substances are either preformed and granule associated (e.g., histamine, neutral proteases, proteoglycans) or produced during activation of the cell (e.g., prostaglandin D2, leukotrienes and other metabolites of arachidonic acid, and platelet-activating factor). Appropriately stimulated mouse or human mast cells can release the cytokine tumor necrosis factor α (TNF-α),52,53 and many other cytokines, chemokines, and growth factors with effects on inflammation, immunity, hematopoiesis, tissue remodeling, and many other biologic processes.1,3,4,5,49,50,51 By contrast, the spectrum of basophil-derived cytokines appears to be more limited but includes IL-4 and IL-13, vascular endothelial growth factor (VEGF)-A,54 certain chemokines, and, at least in mice, IL-6, TNF-α, and thymic stromal lymphopoietin (TSLP).4,5,55,56,57
Basophils and mast cells have specific, high-affinity plasma membrane receptors for the Fc region of IgE (FcεRI).58,59 When IgE antibodies bound to FcεRI on the basophil or mast cell surface are bridged by specific divalent or multivalent antigens, anaphylactic degranulation is triggered.57,58 The critical signal in this event is the aggregation of FcεRI on the plasma membrane.57,58 Anaphylactic degranulation involves the fusion of plasma membranes with the membranes delimiting individual cytoplasmic granules or with groups of granules whose membranes have undergone fusion, leading to rapid noncytolytic release of granule contents, such as histamine and other preformed mediators.1,2 The complex sequence of biochemical events associated with anaphylactic degranulation, the signaling mechanisms that positively and negatively regulate this response, and the rationale for the pharmacologic manipulation of these processes have been reviewed.57,58
The sudden, massive release of mediators from basophils and mast cells provokes many of the clinical manifestations of acute immediate hypersensitivity reactions in disorders such as certain forms of bronchial asthma (including fatal asthma, in which basophils can be prominent60); urticaria; allergic rhinitis; and anaphylaxis to foods, drugs, insect stings, and other antigens.1,5,49,50,55,56,57 Both mast cells and basophils can bind IgG antibodies (as well as IgE), and the binding of IgG or IgG immune complexes can contribute to the activation of these cells (in certain mouse models of anaphylaxis) or, in other circumstances, to the downregulation of their activation (e.g., via FcγRIIB).5,55,56,57,61,62,63,64 Other diverse stimuli, including certain complement fragments (anaphylatoxins), neutrophil lysosomal proteins, a variety of basic peptides and peptide hormones, components of insect or reptile venoms, radiocontrast solutions, cold, calcium ionophores, and certain drugs such as narcotics and muscle relaxants, also may initiate rapid release of mediators from basophils and/or mast cells, independently of IgE.1,4,5,48,49,54,55,56 The clinical reactions provoked by these agents can closely mimic those of immediate hypersensitivity. Basophils activated via FcεRI or other mechanisms can exhibit increased surface levels of CD63, CD69, and CD203c, and the clinical value of using such findings to monitor basophil activation in vivo (e.g., in the setting of allergic disorders or antigen-specific immunotherapy) is under investigation.65
Mast cells and basophils also can contribute to late-phase reactions. Late-phase reactions occur when antigen challenge is followed, hours after initial IgE-dependent mast cell activation, by recurrence of signs (e.g., cutaneous edema) and symptoms (e.g., bronchoconstriction).49,50 Much of the morbidity associated with chronic allergic conditions, including allergic asthma, is widely believed to reflect the actions of leukocytes that are recruited to sites of late-phase reactions.49,50 Studies in mast cell–deficient and mast cell knockin mice (genetically mast cell–deficient mice that have been selectively repaired of their mast cell deficiency) indicate mast cells are responsible for virtually all of the vascular permeability changes and leukocyte infiltration associated with IgE-dependent cutaneous late-phase reactions66,67 and that TNF-α can, importantly, contribute to these responses.66 The extent to which mast cells (or TNF-α) contribute to late-phase reactions in humans, in which such reactions may have components that are either IgE or T-cell dependent (and in which it has been suggested that certain IgE-dependent mechanisms may not involve mast cells63), is not yet clear.68,69 However, the lymphocytes, basophils, eosinophils, and other leukocytes that are recruited to these reactions likely produce cytokines and other mediators that regulate further development and, ultimately, resolution of these reactions.4,5,49,50
Studies in mice (see “Genetic Approaches for Analyzing Basophil and Mast Cell Function” below) indicate that mast cells can contribute importantly to many of the features of asthma, as observed in certain mouse models of chronic allergic inflammation involving the lungs. The features include the development of airway hyperreactivity to immunologically nonspecific agonists of bronchoconstriction such as methacholine; infiltration of the airways and lung interstitium with inflammatory cells, including eosinophils, neutrophils, and T cells; increased deposition of collagen in the lungs and hyperplasia and/or hypertrophy of airway smooth muscle; and induction of increased numbers of mucus-producing goblet cells in the large airways.50,70,71 Thus, such mouse models indicate mast cells and their products can promote the development of much of the pathology and pathophysiologic changes observed in longstanding asthma in humans.50,70,71 Studies in mice indicate that basophils also can enhance the development of IgE-dependent chronic inflammation of the skin, even in the absence of mast cells or T cells.56,67
As plasma levels of IgE increase (as often occurs in subjects with allergic diseases or parasite infections), levels of FcεRI expression on the surface of basophils and mast cells also increase.72,73 Compared with cells with low “baseline” levels of FcεRI expression, such cells can bind more IgE, release mediators in response to lower concentrations of allergens, and produce significantly larger amounts of preformed and lipid mediators and cytokines.74 Thus, basophils and mast cells in subjects with high levels of IgE may have significantly enhanced ability to express IgE-dependent and/or immunoregulatory functions.49,74 Exposure of mouse mast cells to certain monoclonal IgE antibodies, in the absence of exposure to the antigen for which the IgE is known to have specificity, can enhance the survival of the cells and, in some cases, induce the cells to release all three classes of mediators (preformed, lipid, cytokines).74 Exposure to IgE in the absence of known antigen also can induce enhanced survival and cytokine and chemokine release in in vitro derived human mast cells.75 Although the mechanisms responsible for these intriguing findings are not fully understood, some types of IgE antibodies appear to induce aggregation of FcεRI in the absence of known antigen.74,76 The clinical implications of these findings, if any, remain to be defined.
Mast cell activation and/or infiltration of affected tissues with circulating basophils can occur during a variety of T-cell–dependent immunologic responses in both humans and experimental animals.1,5,49,55,56,57,63,64,77,78,79 However, despite years of study, the importance of the contributions of mast cells or basophils in such settings is not fully understood. In part this may reflect differences in the types of mast cell–deficient mice used to investigate such responses and/or the strain background of such mice, and in part this may reflect differences in the details of the experimental models used to probe the roles of mast cells and basophils in these settings (see “Genetic Approaches for Analyzing Basophil and Mast Cell Function” below). In some of the models of T-cell–dependent responses tested, authors have concluded that mast cell can either enhance or suppress features of the responses, and in some settings evidence has been reported that mast cells can enhance the features of relatively weak responses and suppress features of strong responses.63,64 It also has been proposed that mast cells and basophils might have immunomodulatory effects on the development or magnitude of certain acquired immune responses (e.g., positive effects via mast cell–dependent enhancement of dendritic cell migration or activation or negative effects via the production of IL-10 by mast cells).5,55,56,57,63,64,78,79,80 However, some of the findings in this area are considered controversial.5,63,64,79
Basophils and mast cells may have critical roles in the expression of host resistance to certain parasites. Whether basophils, mast cells, or both represent major effector cell types in these responses appears to vary according to factors such as species of parasite, species of host, and site of infection. Thus, in the guinea pig, basophils appear to be required for expression of immune resistance to infestation of the skin by larval ixodid Amblyomma americanum ticks,77,81 whereas expression of IgE-dependent immune resistance to the cutaneous infestation of larval Haemaphysalis longicornis ticks in mice is dependent on mast cells and basophils, and IgE.57,82 Such findings support the notion that basophils and mast cells express similar or complementary functions as effector cells in host defense against parasites and other agents.
Studies in mast cell–deficient and “mast cell knockin mice” (see “Genetic Approaches for Analyzing Basophil and Mast Cell Function” below) or in mice that lack TNF-α or certain mast cell–associated proteases indicate that mast cells can contribute to “innate” host defense against some experimental bacterial infections.63,64,83 Depending on the model system tested, the beneficial role of the mast cell in such models of “innate immunity” in mice may partly reflect complement-dependent, toll-like receptor (TLR) 4-dependent, endothelin-1–dependent, or neurotensin-dependent activation of mast cells, inducing the release of mast cell–derived mediators, which, in turn, can contribute to enhanced local recruitment or activation of neutrophils and enhanced clearance of bacteria, or which may reduce harmful levels of TNF-α.63,64,83 Studies in mice indicate mast cells can phagocytose bacteria,83 and mouse and human mast cells can produce antimicrobial peptides (cathelicidin LL-37 in humans).84 However, mast cells also may contribute to survival during bacterial infection in mice by additional mechanisms, such as protease-dependent degradation of endothelin-1,85,86 neurotensin,87 and perhaps other endogenous peptides that are produced during infections and which contribute to the pathology associated with the disorders.63 On the other hand, some mast cell functions that may be expressed during bacterial infections in mice, such as the ability of mast cells to degrade IL-6 via dipeptidyl peptidase 188 or to produce IL-10 that suppresses host acquired immunity,89 may have detrimental consequences. Thus, mast cells may have complex roles in innate immune responses, with some actions promoting host defense and survival and others enhancing the pathology associated with the response.
Mast cell progenitors90,91,92 and basophils93 can become infected with “M tropic” strains of HIV. Although mature mast cells appear to be resistant to such infection, mast cells that matured from infected progenitors while harboring latent infection exhibited enhanced viral replication upon stimulation with ligands for TLR2, TLR4, or TLR9,91,92 or via an IgE-dependent mechanism.92 At least one HIV-derived protein, glycoprotein 120 (gp120), can induce mast cell or basophil mediator release (histamine and, in basophils, IL-4 and IL-13) by binding to and crosslinking cell-surface-bound IgE.93 Many patients infected with HIV develop high levels of IgE and can exhibit exacerbation of the symptoms and signs of their allergic disorders.93 However, the clinical significance of such findings in HIV-infected patients remains to be determined. Many potential secreted products of mast cells or basophils may have effects that enhance (or suppress) host responses to a variety of viruses or contribute to the pathology associated with the infections.94 However, the extent to which mast cells contribute to host defense or pathology during viral infections is not clear.
The venoms of many animals contain substances that can activate mast cells via innate mechanisms and/or can induce specific IgE responses to venom components.95,96 Many humans have IgE antibodies against components of honeybee or wasp venom, but only a small fraction of such “venom-sensitized” individuals have a history of anaphylaxis or other serious clinical response to such venoms.97 Work in mast cell–deficient, mast cell knockin, and mast cell protease-deficient mice indicates that mast cells can enhance the resistance of mice to diverse animal venoms and/or their toxic components, including the venoms of three poisonous snakes,96 the honey bee,96,98 the Gila monster,99 and two species of scorpions,99 as well as to sarafotoxin 6b, a major toxin in Israeli mole viper snake venom86 and helodermin, a toxin in Gila monster venom.99
Notably, carboxypeptidase A3 (CPA3) or mouse mast cell protease (mMCP-4, the functional counterpart in the mouse to human chymase) appeared to account for much or all of the protective effects against various venoms that were attributable to mast cells.86,96,98,99 CPA3 and mMCP-4 can degrade both endogenous biologically active peptides (endothelin-1 [ET-1]86,96 and vasoactive intestinal polypeptide [VIP],99 respectively) and similar peptides present in animal venoms (sarafotoxin 6b and helodermin, respectively), which are thought to act in mammals via the same receptors that bind the similar endogenous peptides. Work in mice deficient in mast cells, IgE, or components of the FcεRI suggests that mast cells also can contribute to the IgE antibody- and FcεRI-dependent enhanced resistance to challenge with potentially lethal amounts of honeybee venom that is observed in animals after an initial exposure to a sublethal amount of that venom.98 Indeed, it has been hypothesized that the ability to participate in innate and acquired immune defenses against components of venoms and other toxins are important functions of mast cells and, in the case of acquired immunity, of IgE antibodies.95,96,100
Factors capable of inducing basophil infiltration, mast cell proliferation, and/or basophil or mast cell activation are generated during a wide variety of immunologic or pathologic processes, including immune responses to parasites.1,5,30,49,50,51,55,56,57,77 As a result, speculation is considerable that basophils and mast cells express critical roles in diverse biologic responses. On the other hand, the precise functions of basophils and mast cells in most of the biologic responses in which the cells have been implicated are obscure. Studies of basophil function in guinea pigs81 and mice55,56,57 have employed antibodies to deplete basophils, and such approaches also have been used to deplete mast cells in mice101; however, the antibodies used may also influence other cell types.5,55,56,57,63,64,79 Various mutant or transgenic mice that exhibit constitutive or inducible depletion of some or all mast cell populations, as well as mast cell–deficient mice that have been selectively engrafted with wild-type or genetically altered mast cells (so-called mast cell knockin mice), have been used in efforts to define and quantify the contributions of mast cells to many different biologic responses.63,64,79 Transgenic mice also are now available that exhibit constitutive or inducible depletion of basophils, or that lack certain mast cell–associated products either globally or in selected cell populations.5,55,56,57,63,64,79 As has been extensively reviewed,5,54,55,56,74,75,76 the availability of such models has the potential to advance substantially our understanding of the roles of mast cells and basophils in health and disease in mice, including ascertaining the importance of strain background on some of the findings. In evaluating the results of such studies, one should keep in mind that such genetic models can have various advantages and disadvantages, and that even very strong evidence that mast cells or basophils have particular roles in mice does not prove that the cells have identical functions in humans.
No humans devoid of mast cells have been reported. In addition, the clinical findings in the rare patients who express a deficiency of basophils are not easy to interpret. One patient with a profound basopenia experienced persistent and severe infestation with scabies,32 a finding that might be viewed as consistent with the role of basophils in resisting ectoparasites in humans. However, that patient also had eosinopenia, IgA deficiency, and multiple other clinical problems.32 A second basophil-deficient patient had a history of recurrent bacterial and viral infections.34 However, this patient also had a deficiency of eosinophils, hypogammaglobulinemia, abnormal suppressor T-cell function in vitro, and a thymoma.34
BLOOD BASOPHIL COUNT
The normal blood basophil count is difficult to define precisely, but several studies place the normal range between approximately 14 to 20 and 80 to 90/μL (approximately 0.014 to 0.020 and 0.080 to 0.090 × 109/L).8,9,10,102 The blood basophil count reportedly varies by age,103 gender (in one study103 but not in another9), and season.104
Because numbers of blood basophils can be very low even in apparently normal individuals,8,9,10,102 determining whether examples of basophilopenia reflect pathologic processes as opposed to normal variation can be difficult. Nevertheless, reduced numbers of circulating basophils have been reported in several disorders (Table 63–2). Basophilopenia has been recorded in association with urticaria and anaphylaxis,105,106 but the extent to which the latter finding represents a loss of metachromatic staining of circulating degranulated cells rather than a true decrease in the number of cells is undetermined. Basophilopenia occurs in conditions that also are associated with eosinophilopenia. These conditions often are associated with increased secretion of adrenal glucocorticoids.102,107,108 Basophil counts may diminish, sometimes markedly, during leukocytosis accompanying infection, inflammatory states, immunologic reactions, neoplasia, or hemorrhage.107 Basophil counts also are diminished in thyrotoxicosis or after pharmacologic administration of thyroid hormones. Conversely, basophil counts may be increased in myxedema or after ablation of thyroid function.107 A rapid and significant drop in blood basophil levels of up to 50 percent has been documented at ovulation.108 A few patients with an apparent total lack of basophils have been reported.32,34
|