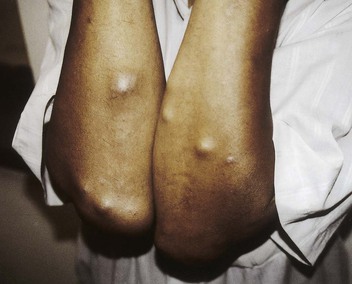
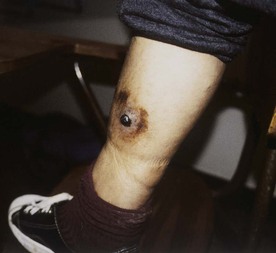
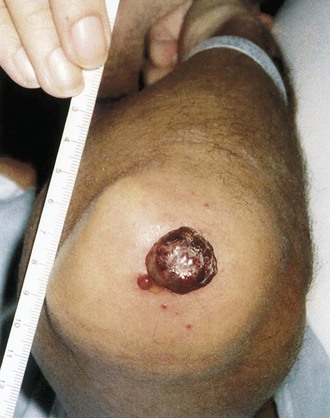
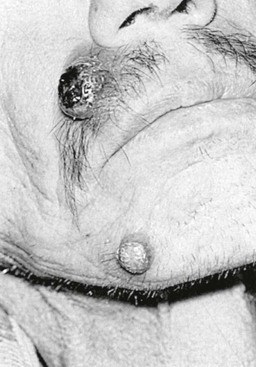
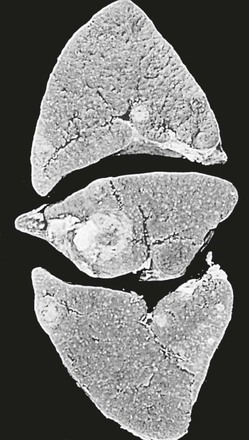
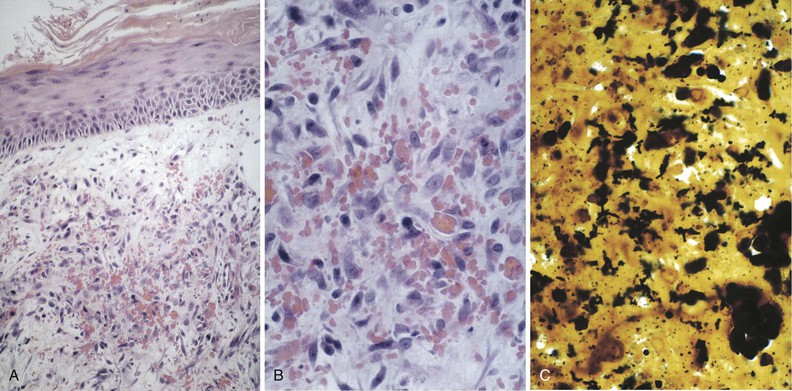
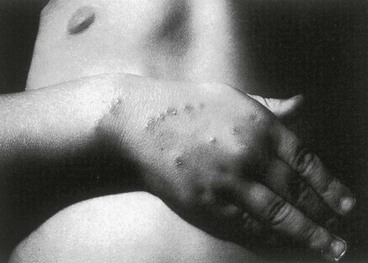
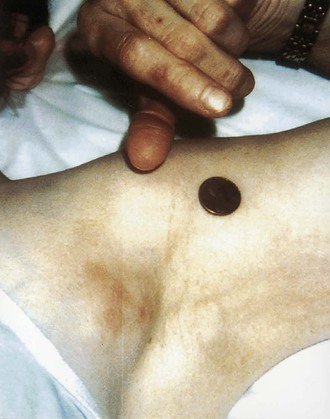
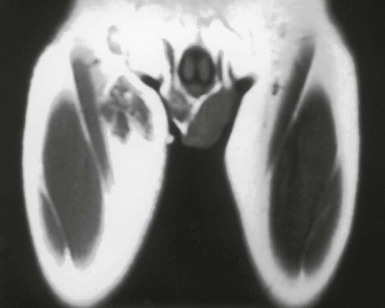
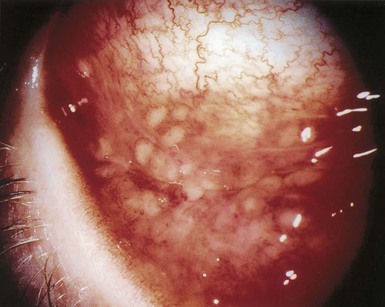
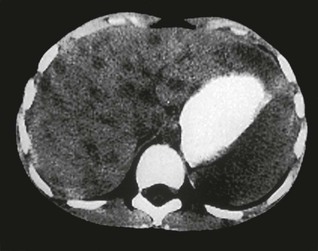
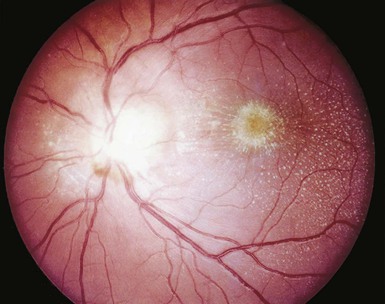
Tejal N. Gandhi, Leonard N. Slater, David F. Welch, Jane E. Koehler The genus Bartonella is a member of the class Alphaproteobacteria and family Bartonellaceae, and closely related to the genera Brucella and Agrobacterium; members of the family Rickettsiaceae are more distantly related. On the basis of genetic similarity,1,2 unification of the genera Bartonella and Rochalimaea as a single genus and the removal of the family Bartonellaceae from the order Rickettsiales were put forth in 1993.2 The similarity of Bartonella to the pathogen Brucella has been further substantiated through whole genome sequencing showing that Bartonella contains a reduced version of the chromosomal elements of Brucella melitensis.3 The genus Bartonella, synonymous with Bartonia, was described in 1913 and referred to the human erythrocyte-adherent organisms originally described by Dr. A. L. Barton in 1909.4,5 The type species is Bartonella bacilliformis. Limited to the Andes mountain regions of South America, B. bacilliformis infection received little attention outside its endemic zone until related bacteria, originally classified in the genus Rochalimaea, were found to be pathogens in individuals with acquired immunodeficiency syndrome (AIDS) in the early 1990s. The former genus Rochalimaea, previously grouped with Bartonella in the order Rickettsiales, had long contained only two member species: Rochalimaea vinsonii, the “Canadian vole agent,” and Rochalimaea quintana (other synonyms: Rickettsia quintana, Rickettsia pediculi, Rickettsia wolhynica, Rickettsia weigl, Burnetia [Rocha-limae] wolhynica, and Wolhynia quintanae),6,7 the agent of trench fever, a debilitating but self-limited human illness so named after it affected many military personnel in World War I.8 Except for sporadic outbreaks, trench fever had all but disappeared from the clinical scene in recent decades. However, R. quintana reemerged in the 1990s as a pathogen of considerable interest9–13 coincident with the discovery of two related species pathogenic to humans, originally named Rochalimaea henselae and Rochalimaea elizabethae.14–17 In 1995, a further merger of a number of species of the genus Grahamella, which are intraerythrocytic bacteria infecting rodents, birds, fish, and other animals, into the genus Bartonella took place.18 Additional species and subspecies have since been identified,19–24,25–28 and some not previously recognized as human pathogens are being found to be infrequent causes of infections in humans.29–31 Although Bartonella spp. have been characterized recently as “emerging” pathogens, DNA analysis of dental pulp provides evidence that Bartonella henselae and Bartonella quintana have existed since antiquity.32,33 A list of validated and additional, recently described34–38 members of the genus Bartonella is provided in Table 236-1. This is an area of active investigation and subject to change. A continuously updated list of validated Bartonella species can be found at www.bacterio.cict.fr/b/bartonella.html. Bartonella species are primarily infectious agents of nonhuman animals. Humans are incidental hosts in most cases (even though animal hosts of B. bacilliformis have not yet been identified), with transmission via inoculation of Bartonella-infected feces from arthropod vectors into nonintact skin. The exception is B. quintana for which the definitive mammalian reservoir is believed to be the human. Presumably because of the limited distribution of sand fly vectors (genus Lutzomyia [formerly Phlebotomus]), natural transmission of B. bacilliformis infection is mainly confined to the Andes mountain range in Peru, Ecuador, and Colombia, at altitudes between 500 and 3200 m.39 Even in the modern antibiotic era, focal outbreaks continue.39 B. quintana is globally distributed, and the only identified vector of B. quintana is Pediculus humanus, the human body louse. Outbreaks of trench fever (also known as Wolhynia fever, Meuse fever, His-Werner disease, shin bone fever, shank fever, and quintan or 5-day fever) have been focal and widely separated. Clusters of trench fever cases are often associated with conditions of poor sanitation and personal hygiene, and they are significantly associated with exposure to body lice. No nonhuman vertebrate reservoirs have been identified for B. bacilliformis. B. quintana has been isolated from nonhuman primates in research colonies in China, but it is not known whether nonhuman primates are infected in the wild.40 B. henselae is globally endemic; serologic studies indicate that infection of domestic cats is worldwide, with the prevalence of antibodies being higher in warm, humid climates, where fleas are more prevalent. Free-ranging and captive wild felids in California also have a substantial prevalence of antibodies reactive with B. henselae,41 and B. henselae has been isolated from the blood of free-ranging mountain lions (Puma concolor) and bobcats (Lynx rufus) in California.42 Prevalence of bacteremia in cats varies43–46 but tends to be higher among feral animals in any particular locale. The B. henselae colony-forming units (CFUs)/mL of blood in infected cats can reach extremely high levels of 106 or more, yet these cats are asymptomatic. B. henselae bacteremia has been documented in healthy domestic cats that have been specifically associated with bacillary angiomatosis (BA)44 or typical cat-scratch disease (CSD)45,47 in their human contacts. Transmission of B. henselae to humans has been linked to cats by serologic and epidemiologic studies.48–50 Diagnosis of CSD has been confirmed in the patients by culture recovery of B. henselae from lymph nodes with the pathologic findings of CSD lymphadenitis45,51 and by detection of B. henselae DNA by polymerase chain reaction (PCR) in tissue from cases of CSD lymphadenitis52,53 and conjunctival disease,54 as well as in CSD skin test antigen.55,56 The major arthropod vector of B. henselae is the cat flea, Ctenocephalides felis, as evidenced by epidemiologic associations,45,48,50 identification of B. henselae by culture and DNA amplification from cat fleas,43,44 and transmission of B. henselae among cats by infected fleas under controlled experimental conditions.57 Cat fleas appear to serve as efficient vectors for cat-to-cat transmission43; their contribution to human infection likely involves inoculation of Bartonella-infected flea feces into the human during a cat scratch. Additional Bartonella species have also been identified in cat fleas.58 Other types of fleas, as well as ixodid and Dermacentor ticks, have been found to harbor various Bartonella species, but transmission to humans via ticks has not yet been demonstrated.59,60–64 Bartonella clarridgeiae19,20,22 and Bartonella koehlerae64 also cause asymptomatic, persistent bloodstream infection in cats. Although B. koehlerae is infrequently isolated from domestic cats, B. clarridgeiae is quite prevalent, especially in European domestic cats.20,65 Of 50 Bartonella isolates recovered from 94 stray cats in France, Heller and colleagues20 found that 34% were B. henselae genotype I, 36% were B. henselae genotype II, and 30% were B. clarridgeiae. B. clarridgeiae was present in the blood of 16% of the stray cats. Despite the high prevalence of B. clarridgeiae in cats, this species has neither been isolated from, nor DNA amplified from, an immunocompromised or immunocompetent human. Bartonella koehlerae is occasionally transmitted to humans and represents an uncommon cause of illness.30 Because B. elizabethae is rarely identified in humans, little is known of its epidemiology, except that it has been cultured from the blood of rodents66 and its DNA has been amplified from the blood of rodents,66 fleas from rodents,66 and dogs.67 The long-suspected link between Oroya fever and verruga peruana was confirmed tragically in 1885 by Daniel Carrión, a medical student who injected himself with blood from a verruga peruana lesion and subsequently died of “Oroya fever.”68 The eponym “Carrión’s disease” has since denoted the full spectrum of B. bacilliformis infection. Oroya fever, an acute hematologic disease resulting from primary bacteremia and erythrocyte invasion, develops 3 to 12 weeks after inoculation.69 In its mildest, insidiously developing form, a febrile illness can last less than a week and may go unrecognized and give rise to subsequent cutaneous manifestations that are the first recognized clinical findings.70–72 When illness is abrupt in onset, high fever, chills, diaphoresis, anorexia, prostration, headache, and mental status changes are associated with rapidly developing, profound anemia resulting from bacterial invasion of erythrocytes, causing shortened life span and destruction of erythrocytes.72–74 Intense myalgias and arthralgias, abdominal pain and emesis, jaundice, lymphadenopathy, thrombocytopenia, and complications such as seizures, delirium, meningoencephalitis, obtundation, dyspnea, hepatic/gastrointestinal dysfunction, and angina pectoris can occur during this stage,72,75,76 most believed to be a consequence of the anemia and of microvascular thrombosis, resulting in end-organ ischemia. Without antimicrobial therapy, the fatality rate is high for the severe, abrupt form of hematic illness.75 With appropriate treatment in the modern era, mortality is reported to be less than 10%.72 For survivors, convalescence is associated with a decline of fever and disappearance of bacteria on blood smears but also a temporarily increased susceptibility to subsequent (opportunistic) infections such as salmonellosis72,76,77 or toxoplasmosis.72,78,79 Asymptomatic persistent bacteremia with B. bacilliformis infection can occur in up to 15% of survivors of acute infection.80 These survivors may serve as the reservoir for B. bacilliformis. Whereas some modern era reports paint a picture of similar patterns of recognized disease,72 others suggest that initial infection may more often be asymptomatic or milder than was previously believed.71,81 The eruptive phase of B. bacilliformis infection, “verruga peruana” lesions, usually becomes evident within weeks to months after resolution of untreated acute infection. This late-stage manifestation is characterized by crops of skin lesions marked by an evolution of stages72,73: miliary, then nodular (Fig. 236-1), then what are called mulaire lesions (Fig. 236-2). Mulaire lesions are the most superficial and obviously vascular of the eruptive manifestations, often bulbous, engorged with blood, and prone to ulceration and bleeding. Mucosal and internal lesions can also occur. The nodules may develop at one site while receding at another. Healing at a particular skin site is often punctuated by recurrences and usually takes place over several weeks to 3 or 4 months. Subsequently, fibrosis of mulaire lesions may occur. Histology of active lesions demonstrates neovascular proliferation with occasional bacteria evident in interstitial spaces. Bacterial invasion of/replication within endothelial cells (long believed to be the cause of cytoplasmic inclusions first described by Rocha Lima) is actually rare.82 Acute mortality resulting from bacteremia with non-bacilliformis Bartonella species, even when persistent, is uncommon in immunocompetent hosts. In recent years, B. quintana bacteremic infection outside of the context of human immunodeficiency virus (HIV) infection has been identified sporadically and in small clusters, mainly in homeless people in North America and Europe.13,83,84 “Trench fever” is characterized by a spectrum of self-limited fever often with a 5-day periodicity.7,8 The incubation period may span 3 to 38 days before the usually sudden onset of chills and fevers. In the shortest form, a single bout of fever lasts 4 or 5 days. In the more typical periodic form, there are three to five, and sometimes up to eight, febrile paroxysms, each approximately 5 days apart. Patients are often asymptomatic between febrile paroxysms. The continuous form is manifested by 2 or 3 weeks, and up to 6 weeks, of uninterrupted fever. Afebrile infection is common; one study found 8 of 10 homeless individuals with active B. quintana bacteremia were afebrile.85 Other nonspecific symptoms and signs such as headache, vertigo, retro-orbital pain, conjunctival injection, nystagmus, myalgias, arthralgias, bone pain, hepatosplenomegaly, rash, leukocytosis, and albuminuria may accompany B. quintana bacteremia. B. henselae bacteremia in HIV-uninfected people is rare and may present with abrupt onset of fever, which may persist or become relapsing. Localizing symptoms or physical findings are unusual.14,16,86,87 Aseptic meningitis concurrent with B. henselae bacteremia has been documented.87,88 B. quintana (and rarely B. henselae) bacteremia has the potential to evolve into long-term persistence if not treated appropriately.84,87 HIV-infected patients with advanced immune suppression (CD4+ cell count <100 cells/mm3) are more likely to develop severe and prolonged infection from B. quintana or B. henselae.89,90 Bartonella bacteremia in HIV-infected people has been associated with cutaneous bacillary angiomatosis (B. henselae or B. quintana), hepatic and splenic bacillary peliosis (B. henselae), granulomatous hepatitis (B. henselae), fever of unknown origin (FUO) (B. quintana and B. henselae), and occasionally endocarditis (B. quintana much more frequently than B. henselae).10,86,89,90 In a study of 49 patients with bacillary angiomatosis-peliosis (92% had HIV infection), 50% of patients sampled had bacteremia with either B. quintana or B. henselae.91 The clinical presentation is often characterized by insidious development of malaise, body aches, fatigue, weight loss, progressively higher and longer recurring fevers, and sometimes headache.90 Hepatosplenomegaly or splenomegaly may occur, but localizing symptoms or physical findings are often lacking. B. quintana and B. henselae are recognized as important causes of blood culture–negative endocarditis.10,12,92–99 Two large European studies of patients with blood culture–negative endocarditis found Bartonella species to be the second most frequent identifiable cause following Coxiella burnetii.98,99 In the United States, where Coxiella endocarditis is less common, Bartonella species may be the most common cause of culture-negative endocarditis. People with B. quintana endocarditis are usually homeless, often substance abusers, and have exposure to body lice, whereas people with B. henselae endocarditis more commonly have had cat exposure and preexisting valvular heart disease.98 In a retrospective study of 101 patients with Bartonella endocarditis,97 presentation was usually subacute and a significant proportion (17%) of patients were afebrile at the time of presentation. Embolic phenomena were reported in 44 (43%) of the patients on presentation. Fifty-eight patients (57%) had previously known valvular heart disease; 58 (57%) of the patients had involvement of the aortic valve; and 18 (18%) had involvement of multiple valves. Irrespective of antimicrobial therapy, 76 patients had severe valvular damage and required valvular surgery. Twelve patients ultimately died; 2 were cured only after treatment for a relapse, and the remaining 87% were cured with the initial therapy. The diagnosis in blood culture–negative cases has been established with serology, DNA amplification from blood or valve tissue, immunohistochemistry, or all three. Patients with B. henselae and B. quintana endocarditis have had clinical features resembling a systemic vasculitis associated with a positive cytoplasmic-antineutrophil cytoplasmic antibody.92,100,101 In these patients, detection of Bartonella infection is critical to avoid inappropriate initiation or continuation of immunosuppressive therapy. Antiproteinase 3 antibodies have also been found in patients with Bartonella endocarditis and associated glomerulonephritis.101a Despite the pediatric predominance of CSD, pediatric B. henselae endocarditis has been recognized only rarely.96 This is likely due to the infrequency of bacteremia due to B. henselae, compared with B. quintana, and the infrequent exposure of children to risk factors for B. quintana: homelessness and body lice. B. elizabethae has been isolated once as the cause of bacteremia and endocarditis and in a case of clinically suspected Oroya fever.17,101b B. vinsonii is generally not considered a human pathogen, although B. vinsonii subsp. arupensis and B. vinsonii subsp. berkhoffii have been associated with isolated cases of endocarditis in humans.26–28 B. vinsonii subsp. arupensis was also the cause of bacteremia and fever in an otherwise healthy rancher from the western United States.25 B. vinsonii subsp. berkhoffii can also cause bacteremia and endocarditis in dogs.23,24 In addition, Bartonella koehlerae and Bartonella alsatica have been associated with endocarditis in humans.29,30,102 Wild rabbits (a known reservoir of B. alsatica) were a possible source of infection in two patients with B. alsatica endocarditis.29,102 Bartonella rochalimae caused a febrile, bacteremic illness in a traveler returning from Peru who had sustained numerous arthropod bites.34 B. rochalimae has now been isolated from gray foxes (Urocyon cinereoargenteus) in northern California, implying a canid reservoir.35 Bartonella tamiae was isolated from the blood of three febrile residents of Thailand. BA (also referred to as epithelioid angiomatosis or bacillary epithelioid angiomatosis) is a disorder of neovascular proliferation originally described involving skin and regional lymph nodes of HIV-infected people with more advanced immune suppression (CD4+ cell count <100 cells/mm3).89,90,103–105 It has been demonstrated subsequently to involve a variety of internal organs, including liver, spleen, bone, brain, lung, bowel, and uterine cervix,86,105–112 and to occur in other immunocompromised86,107,113,114–118 and, rarely, immunocompetent hosts.119,120 B. henselae and B. quintana have been found to be a cause of BA both by direct culture9,16,43,86,121 and by PCR amplification of Bartonella-specific DNA sequences from tissue.* Either species can cause cutaneous lesions, but subcutaneous and osseous lesions are more often associated with B. quintana, and hepatic and splenic bacillary peliosis only with B. henselae.91 In addition, patients with B. henselae genotype I infection may be more likely to have hepatic and splenic peliosis, while patients with B. henselae genotype II are more likely to develop BA lesions involving the skin or lymph node, or both.123 Cutaneous BA lesions often arise in crops, but both the temporal pattern of development and the gross morphologic characteristics can vary. They can be remarkably similar to lesions of verruga peruana or may resemble pyogenic granuloma, but the major clinical differential diagnosis in HIV-infected patients is Kaposi sarcoma.124 In gross appearance, BA skin lesions125 can be subcutaneous, dermal nodules, or single or multiple papules that are flesh colored, red, or purple. Skin lesions may also display ulceration, serous or bloody drainage, and crusting (Figs. 236-3 and 236-4). Lesions can range in diameter from millimeters to centimeters, number from a few to hundreds, be fixed or freely mobile, be associated with enlargement of regional lymph nodes, involve mucosal surfaces or deeper soft tissues, occur in a variety of distributions, and bleed copiously when incised. Visceral lesions can be quite dramatic as well, in both their number and their heterogeneity of gross appearance (Fig. 236-5). When cutaneous lesions are absent, diagnosis is often delayed because the features associated with hepatic or splenic bacillary peliosis (fever, lymphadenopathy, hepatomegaly, splenomegaly, anemia, and serum alkaline phosphatase elevation) are nonspecific.89 BA is distinguished from other neovascular tumors histologically.125,126 It consists of lobular proliferations of small blood vessels containing plump, cuboidal endothelial cells interspersed with mixed inflammatory cell infiltrates having neutrophil predominance (Fig. 236-6A and B). Endothelial cell atypia, mitoses, and necrosis may be present. Fibrillar- or granular-appearing amphophilic material is often present in interstitial areas when stained by hematoxylin and eosin (H&E) stain. Warthin-Starry staining or electron microscopy demonstrates these to be clusters of bacilli (see Fig. 236-6C). Bacillary peliosis (BP), originally described involving the liver and sometimes spleen in HIV-infected people,127 has since been identified in other immunosuppressed people and found to involve lymph nodes as well.86,114,116,128 Molecular epidemiologic investigation has revealed that B. henselae appears to be the only Bartonella species eliciting this host response.91 Involved organs contain numerous blood-filled cystic spaces (peliosis) that can range from microscopic to several millimeters in size. H&E-stained tissue reveals partially endothelial cell-lined peliotic spaces often separated from surrounding parenchymal cells by fibromyxoid stroma containing a mixture of inflammatory cells, dilated capillaries, and clumps of granular material. Such clumps are filled with Warthin-Starry-staining bacilli.127 Presenting clinical features are often nonspecific, although there is a dramatic report of an HIV-infected patient presenting with fever and abdominal pain who developed massive hemoperitoneum from hepatic bacillary peliosis.129 Hepatic and splenic bacillary peliosis appear as multiple hypodense or low attenuation lesions on ultrasonographic or computed tomographic abdominal scanning.130 Inflammatory reactions in immunocompromised hosts caused by B. henselae infection without associated angiomatosis or peliosis have been reported involving liver, spleen, lymph nodes, heart, lung, and bone marrow.131,132 They are characterized by nodular collections of lymphocytes and nonepithelioid histiocytes that may become centrally necrotic, containing aggregates of neutrophils and karyorrhexic debris suggestive of microscopic abscess formation.131,132 These may represent a clinical-pathologic link with CSD and are more likely to occur in HIV-infected patients who have less severe immunosuppression.130 Among the Bartonella species, CSD has been associated nearly exclusively with B. henselae. Evidence indicating its cardinal role includes the serologic responses of people with CSD49,50,133; the identification of B. henselae in CSD lymphadenitis by culture,51 PCR-based DNA amplification,52,53,134–137 and immunocytochemistry138; detection of B. henselae in CSD skin test antigens by PCR55,56; and the recovery of B. henselae from the blood of healthy cats (which can be persistently bacteremic)43,45,139 and from cat fleas.43 The various manifestations comprising CSD have been recognized for more than a century, but “la maladie des griffes de chat” was not defined as a syndrome until 1950.140 CSD remained an infection in search of an agent for more than 40 years after that. Thus, most cases have been identified by clinical/pathologic criteria, supplemented by reactions to unstandardized skin test antigens prior to identification of B. henselae. It is reasonable to ascribe the majority of CSD cases to B. henselae on the basis of the numerous lines of evidence developed in recent years. Yet it remains possible that other agents can very rarely cause “typical” CSD cases, such as have been reported with Afipia felis141,142 and B. clarridgeiae.21 CSD is the most commonly recognized manifestation of human infection with Bartonella. In the United States, estimated CSD cases approach 25,000 annually.143 Interestingly, veterinary care personnel do not have evidence of notably higher levels of infection than the general population.144 “Typical CSD” represents 88% to 89% of cases overall. A primary cutaneous papule or pustule develops approximately 3 to 10 days after an animal contact (most commonly a kitten or feral cat) at a site of inoculation (usually from a scratch or bite) (Fig. 236-7),145–147 and it may last for 1 to 3 weeks. Regional lymphadenopathy ipsilateral to the inoculation site (mainly head, neck, or upper extremity), which develops in 1 to 7 weeks (Figs. 236-8 to 236-10), is the most prominent and common manifestation (>90% of typical cases) and the one that usually precipitates medical evaluation. Even at the time of such presentation, an inoculation site (scratch, bite, or primary papule or pustule) may be detected in more than two thirds of patients when actively sought. One third to 60% of patients may have low-grade fever lasting several days. One fourth of patients report malaise or fatigue, and approximately 10% report headache or sore throat. Transient rash occurs in approximately 5% of patients. Transient mild leukocytosis, with increased neutrophils and sometimes eosinophils, as well as elevated erythrocyte sedimentation rate, may occur. Nearly half of typical CSD patients have single lymph node involvement, another 20% have multiple node involvement at one site, and the remaining one third have node involvement at multiple sites. Up to one sixth of patients with typical CSD develop lymph node suppuration. Ultrasonography may assist in the assessment of lymph node size and suppuration,45,148 and it may be used to direct needle aspiration of pus (usually done to relieve discomfort). Node enlargement usually persists for 2 to 4 months but may last considerably longer; spontaneous resolution is the rule, regardless of whether the patient is treated with antibiotics. The histopathology of nodes includes a mixture of nonspecific inflammatory reactions including granulomata and stellate necrosis. Bacilli are best demonstrated by Dieterle, Warthin-Starry, or Steiner silver staining. Hypercalcemia uncommonly may complicate CSD lymphadenopathy as a result of endogenous overproduction of active vitamin D associated with granuloma formation.149 Previously considered to be uncommon in CSD, musculoskeletal manifestations actually occur in more than 10% of cases, as defined in an 11-year surveillance study involving 913 patients with compatible clinical presentation and confirmatory PCR or serologic test, or both, for B. henselae.150 Myalgia occurred in 5.8%, had a median duration of 4 weeks, and was often severe. Arthropathy (arthralgia or arthritis, or both) occurred in 5.5%, involving mainly the medium and large joints for a median of 5.5 weeks and characterized as moderate to severe in intensity in more than half of these patients. In a small proportion, chronic symptoms persisted for more than 1 year and could be debilitating. Much less commonly, tendonitis, neuralgia, and osteomyelitis were identified, all at a rate of less than 1%. Age older than 20 years increased the risk of having any of these symptoms, and female gender was also associated with increased risk of arthropathy. A review of 47 patients with bone infections associated with CSD found the median age was 9 years. The most frequently involved sites were the vertebral column (42%) and the pelvic girdle (27%).151 Up to half of the overall 11% or 12% of CSD cases that are atypical represent Parinaud’s oculoglandular syndrome, a self-limited granulomatous conjunctivitis and ipsilateral, usually preauricular, lymphadenitis (Fig. 236-11).152,153 Various other “atypical” manifestations154 include self-limited granulomatous hepatitis/splenitis,155 atypical pneumonitis,156 osteitis,157 and neurologic syndromes (mainly encephalopathy and neuroretinitis). A syndrome of prolonged FUO in children has been described as well.158 Because of the insidious and nonspecific nature of the fever and abdominal pain of CSD hepatitis/splenitis, diagnosis may be delayed until a history of cat exposure prompts serologic testing and ultrasonographic or computed tomographic abdominal imaging, which usually demonstrates multiple hypodense lesions (Fig. 236-12).130,159–161 Similarly, the nonspecific nature and rarity of many of the other atypical manifestations except Parinaud’s syndrome may result in delay in their accurate diagnosis until a history of cat exposure or suggestive findings on histopathology prompt specific evaluation directed at B. henselae.145 A dramatic if infrequent manifestation, encephalopathy, was first reported within a few years of the description and naming of CSD.162–164 Encephalopathy probably occurs in 2% to 4% of all CSD cases recognized, although estimates range as widely as 1% to 7%.165 Extrapolating from the estimated U.S. CSD case rate,143 500 to 1000 annual CSD encephalopathy cases occur in the United States. Recognition of this phenomenon may increase with the availability of serologic testing. It remains predominantly a clinical diagnosis, subject to laboratory confirmation by techniques described later (predominantly antibody testing). Adolescents and adults may represent a greater proportion of cases of CSD encephalopathy than they do of CSD overall.166 Although encephalopathy usually follows the development of lymphadenopathy, it has also been reported to precede lymph node involvement or to occur in its absence. Persistent, generalized headache is a common part of the history, but fever is an inconsistent finding. Patients may become restless, and combativeness is often described. Nearly half of patients develop seizures that range from focal to generalized, and from brief and self-limited to status epilepticus. Short-term anticonvulsant therapy may be required, as well as supportive therapy when there is obtundation or coma. Concurrent acute neurologic manifestations may be present transiently (e.g., nuchal rigidity, pathologic reflexes, pupillary dilatation). When they occur, neurologic deficits such as aphasia, cranial nerve palsy, paresis, hemiplegia, and ataxia are also usually self-limited, although time to resolution may span weeks to months to as long as 1 year. However, persistence of intellectual impairment and of seizures has been reported uncommonly.167–169 Laboratory studies, such as cerebrospinal fluid (CSF) analysis and culture, generally do not add specific diagnostic findings to the clinical picture of CSD encephalopathy but, rather, serve to exclude other processes. Elevations of CSF protein concentration and leukocytes occur in only approximately one third of patients (but do not necessarily coincide in the same patients); lymphocytes predominate. Hypoglycorrhachia is rare. CSF cultures have been consistently negative, even since the recognition of the CSD-B. henselae association. Studies of the brain with computed tomography (CT) or magnetic resonance imaging, or both, are usually normal, but a few cases of persistent structural abnormalities have been reported.168,169 Electroencephalography during the acute phase of CSD encephalopathy commonly reveals diffuse slowing, yet another nonspecific feature that resolves with clinical recovery. The pathogenesis of CSD encephalopathy and other central nervous system (CNS) manifestations associated with CSD remains unclear. It is unknown whether these rare complications are attributable to direct invasion of the CNS by B. henselae or to other mechanisms, such as vasculitis or immune response. B. henselae has been shown to infect feline microglial cells in vitro and to survive intracellularly for up to 4 weeks; however, no ultrastructural abnormalities were identified by electron microscopy within the infected brain cells.170 At autopsy of a rare fatality resulting from CSD meningoencephalitis, there was marked cerebral edema with no gross evidence of acute meningitis. Microscopic examination revealed multiple granulomatous lesions, as well as meningitis and encephalitis. Warthin-Starry silver stain of the brain and liver revealed pleomorphic rod-shaped bacilli consistent with B. henselae. Analysis of brain tissue with PCR confirmed the presence of B. henselae DNA.171 Another such rare case report of the findings in a 6-year-old child who died with disseminated CSD with encephalitis demonstrated intracerebral histologic findings of perivascular lymphocytic infiltrates and microglial nodules.172 Ocular manifestations of CSD include Parinaud’s oculoglandular syndrome (an atypical form of CSD), neuroretinitis, vascular-occlusive events, retinitis, choroiditis, and optic nerve granuloma.173 Neuroretinitis associated with CSD88,166,174,175 has been confirmed to be associated with B. henselae.88,173,176 After its description in 1970,174 neuroretinitis remained solely a clinical diagnosis for years,56 but current culture and nonculture techniques for identification of B. henselae have improved diagnostic accuracy. Bartonella grahamii has been identified once by PCR amplification and sequence analysis in the intraocular fluid of an HIV-seronegative patient with bilateral neuroretinitis and behavioral changes; B. elizabethae has been implicated as the pathogen in another patient with neuroretinitis by serologic antibody studies exclusively.177,178 Neuroretinitis manifests as fairly sudden loss of visual acuity, usually unilaterally, sometimes preceded by an influenza-like syndrome or development of unilateral lymphadenopathy. The most striking, if not most common, retinal manifestation is papilledema associated with macular exudates in a star formation (Fig. 236-13), first associated with CSD in 1984.175 Although this manifestation is characteristic of CSD neuroretinitis, other types of inflammation have also been reported. In a retrospective study of 24 CSD patients with 35 affected eyes, isolated foci of retinitis or choroiditis were the most common ocular manifestation, identified in 83% of eyes and 83% of patients. Optic disk swelling was the second most common finding (46% of eyes and 63% of patients), followed by a macular star (43% of eyes and 63% of patients) and vascular-occlusive events (14% of eyes and 21% of patients). Final visual acuity was 20/25 or better in 26 of 35 eyes (74%) and was similar in both treated and untreated patients.173 Neuroretinitis usually spontaneously resolves with a favorable visual outcome, and the utility of antimicrobial or corticosteroid therapy, or both, remains debated.176 Prospective follow-up of recently well-documented cases reveals that some have mild residual visual deficits.88,176 The differential diagnosis of typical CSD includes many causes of (unilateral) lymphadenopathy, among which are typical or atypical mycobacterial infection, tularemia, plague, brucellosis, syphilis, sporotrichosis, histoplasmosis, coccidioidomycosis, toxoplasmosis, infectious mononucleosis syndromes, lymphoma, and other neoplasms. In solid organ transplant recipients, post-transplant lymphoproliferative disease would also be included in the differential diagnosis. In the inguinal area, tender adenopathy in the absence of a genital lesion suggests Staphylococcus aureus, CSD, lymphogranuloma venereum, and, in the febrile patient with a tick exposure, tularemia. The diagnosis of CSD can be easily overlooked if the clinician fails to obtain an adequate history, especially in the case of the atypical syndromes and not uncommonly in the case of adults with the typical syndrome whose clinicians are inexperienced with CSD. In elderly adults (older than 60 years old), manifestations tend to be less typical, further confounding the diagnosis.179 With domestic cats representing the largest category of companion animals in the United States, the importance of an accurate history regarding animal exposure cannot be emphasized enough when evaluating a patient with findings consistent with CSD. Fortunately, in most cases of CSD, whether typical or atypical, spontaneous resolution occurs. B. henselae or, rarely, B. quintana has been implicated in a small proportion of cases of HIV-associated brain lesions, meningoencephalitis, encephalopathy, and neuropsychiatric disease88,108,180–183 that cannot be ascribed to other causes. A case of intracerebral BA has been described.108 Bartonella infection associated with neurologic manifestations complicating HIV infection has been demonstrated by serum and CSF antibodies and CSF DNA amplification,180 although technical limitations prevented accurate species identification in these cases. A study of autopsy brain tissue reported evidence of B. henselae by immunofluorescence staining and by PCR amplification in three AIDS dementia patients with elevated CSF: serum indices of B. henselae–reactive antibody.182 A nested case-control study reported an association between the presence of serum anti-Bartonella immunoglobulin M (IgM) (implying recent infection) and increased risk of development of neuropsychological decline or dementia.183 At least 4% of new cases of HIV-associated dementia or neuropsychological decline were estimated to result from Bartonella infections and, therefore, to be potentially treatable with antibiotics. Additional case reports have added anecdotal evidence of the utility of antimicrobials in reversing Bartonella-associated neuropsychiatric abnormalities in HIV-infected people.181
Bartonella, Including Cat-Scratch Disease
Background and Classification
Epidemiology of the Common Human-Pathogenic Species
Clinical Manifestations of the Common Human-Pathogenic Species
Oroya Fever and Verruga Peruana: Bartonella bacilliformis
Bacteremic Illness and Endocarditis: Bartonella quintana, Bartonella henselae, and Other Bartonella Species
Other Species
Bacillary Angiomatosis/Peliosis: Bartonella quintana and Bartonella henselae
Cat-Scratch Disease: Bartonella henselae
Background
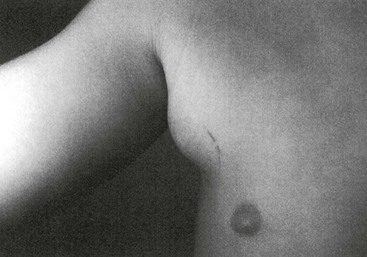
Typical Cat-Scratch Disease
Musculoskeletal Manifestations
Atypical Manifestations
Encephalopathy
Ocular Manifestations
Differential Diagnosis
HIV-Associated Neurologic Syndromes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bartonella, Including Cat-Scratch Disease
236