INDICATIONS FOR AUTOLOGOUS HEMATOPOIETIC CELL TRANSPLANTATION
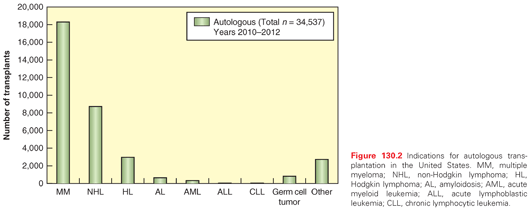
The predominant indication for autologous HCT is the treatment of cancer, although it is sometimes performed for nonmalignant diseases (Fig. 130.2). This chapter focuses on the oncologic indications of autologous HCT.
AUTOLOGOUS HEMATOPOIETIC CELL TRANSPLANTATION FOR PLASMA CELL MYELOMA
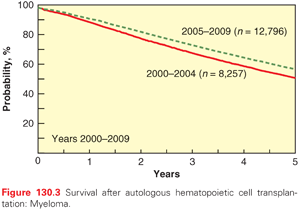
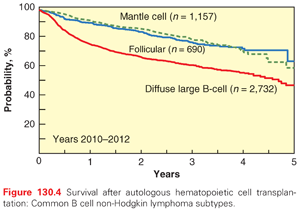
In the United States, myeloma and other plasma cell disorders are the most common indication for autologous HCT (Figs. 130.3 and 130.4). Two large randomized trials11,12 and several nonrandomized comparisons13–15 have demonstrated the efficacy of autologous HCT in prolonging event-free survival (EFS) and overall survival (OS) (in some studies) compared with nontransplant therapies. The majority of autologous HCTs for myeloma are performed as “early or upfront autologous HCT” in newly diagnosed patients after induction therapy. As myeloma is incurable, even those that do not receive an upfront autograft may undergo transplant at relapse (“delayed autologous HCT”). Similarly, those who have benefited from an upfront autograft may receive a second autologous HCT after a subsequent relapse.
Early Experience with Autologous Hematopoietic Cell Transplantation for Myeloma
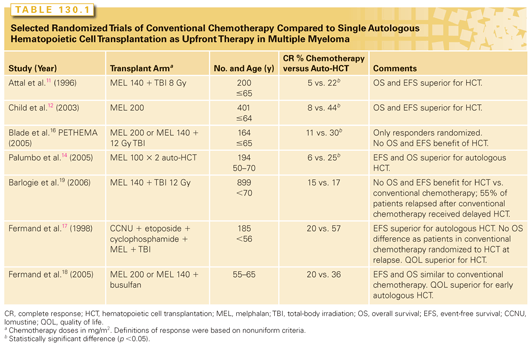
Randomized trials have compared autologous HCT to conventional chemotherapy in newly diagnosed patients with myeloma. The Intergroupe Francophone Myeloma (IFM)11 and Medical Research Council12 led clinical trials that demonstrated that patients randomized to receive autologous HCT had significantly higher complete remission (CR), EFS, and OS rates. Other randomized studies (Table 130.1), however, have not uniformly demonstrated a survival benefit,16–20 although most show an improvement in EFS with the notable exceptions of Blade et al.16 and the US Intergroup study.19 Blade et al.16 randomized patients responding to induction chemotherapy to autologous HCT versus further chemotherapy. The inability to prove an EFS benefit for autologous HCT in this setting may reflect the relatively lower incremental benefit of high-dose therapy in good responders to induction therapy. In the US Intergroup study, 52% of subjects in the chemotherapy arm received a delayed autologous HCT at the time of relapse, which may have masked differences between the two arms.19 A prior randomized French study Myeloma Auto Greffe (MAG 95) reported comparable OS between early versus delayed autologous HCT.17 In general, these studies indicate that autologous HCT improves CR rates and EFS in comparison to conventional chemotherapy. Survival benefit was not seen in studies that permitted or planned for delayed autologous HCT, as well as in studies conducted in later time periods when novel antimyeloma agents were available as an option after relapse.
Conditioning Regimens for Autologous Hematopoietic Cell Transplantation in Myeloma
Several cytotoxic agent combinations and total-body irradiation (TBI) have been evaluated as conditioning regimens for autologous HCT in myeloma. The only randomized phase 3 trial compared intravenous MEL 200 mg/m2 (MEL200) to a combination of MEL 140 mg/m2 (MEL140) and 8 Gy TBI.21 The MEL140-TBI regimen caused significantly more mucositis, delayed hematologic recovery, higher transfusion requirements, and longer hospitalization. OS and progression-free survival (PFS) were similar. Current Center for International Blood and Marrow Transplant Research (CIBMTR) data indicate that most centers (>80%) use MEL200 as conditioning therapy for myeloma. Some groups have attempted to reduce the toxicity of autologous HCT by reducing the dose of MEL to 100 mg/m2 (MEL100). In a randomized study of two planned, sequential autologous HCT procedures performed within 3 to 6 months of each other (e.g., tandem MEL200 versus tandem MEL10022), hospitalization rates, transplant-related mortality (TRM), and OS were similar in both groups, but MEL200 had a superior EFS. MEL200 is considered the standard conditioning before autologous HCT in myeloma.
Older Patients and Those with Comorbidities
The presence of comorbidities and advanced age should be considered when planning autologous HCT for myeloma. While most randomized studies were limited to patients ≤65 years, a survival benefit was shown for MEL100 followed by autologous HCT compared to nontransplant therapy in the 50- to 70-year age group.23 The IFM 99-06 trial arrived at the opposite conclusion in patients aged 65 to 75 years, showing no superiority for MEL100 over MEL-prednisone (MP); in fact, both approaches were inferior when compared with the combination of MP and thalidomide.24 For vulnerable elderly patients or those with organ dysfunction, reduced intensity MEL increases treatment tolerability and optimizes efficacy. MEL140 is the preferred conditioning regimen dose in the elderly population in the United States.
Advanced renal impairment is frequent (20% to 30%) in patients with myeloma, with up to 8% requiring long-term renal replacement therapy.25,26 Autologous HCT is feasible, even in those with advanced renal impairment (defined as a creatinine >3 mg/dl or receiving hemodialysis). MEL200 is associated with severe toxicity, and a dose of 140 mg/m2 is preferred.27,28 Residual DMSO in the thawed infusion can cause toxicity including histamine release, abdominal pain, hypotension, and worsening of renal function. Washing the HPC product to remove DMSO prior to infusion is especially important in the context of renal failure.29
Tandem Autologous Hematopoietic Cell Transplantation
Barlogie and coworkers pioneered the tandem autologous transplant approach in their Total Therapy program.15,30 The IFM94 was the first randomized trial31 comparing single to tandem autologous HCT in patients with previously untreated myeloma. The projected 7-year EFS and OS benefits were significantly better for the tandem autologous HCT arm (10% versus 20% for EFS, and 21% versus 42% for OS, single versus double, respectively). In an unplanned subgroup analysis, there was no benefit for the second autologous HCT for patients who were in a very good partial remission (VGPR) status or better after the first autograft. Another randomized study (Bologna 96) confirmed the observation that the second HCT does not benefit patients in VGPR after the first transplant.32 More importantly, this study did not show an OS benefit for the tandem HCT. Most recently, in a combined Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and the German Multicenter Myeloma Group (GMMG) study (HOVON65/GMMG-HD4), patients receiving tandem autologous HCT in the German (GMMG) component of the study were shown to have superior OS (70% versus 55% at 5 years) over otherwise similarly treated patients receiving a single autologous HCT.33 Randomized studies show tandem autologous HCT produce superior CR rates and EFS overall but not OS (with the exception of IFM94) compared to single autologous HCT. Moreover, benefits of the second autograft appear to apply to patients who are not in a VGPR after the first autologous HCT.
Delaying Autologous Hematopoietic Cell Transplantation until Relapse
Given the incurable nature of myeloma despite novel agents, the timing of autologous HCT (early versus delayed) warrants reappraisal. Randomized studies (from the prenovel drug era) suggest that survival is similar whether autologous HCT is performed early or in the delayed setting after relapse.17,19 Early autologous HCT was associated with a longer treatment-free interval and improved quality of life.17 Boccadaro et al. randomized patients after lenalidomide/dexamethasone induction to tandem autologous HCT or MP/lenalidomide combination. Although CR rates and OS were similar, early HCT provided superior PFS (41 months versus 18 months).34 Data from this trial caution against abandoning the upfront autologous HCT for myeloma. The depth of response and its duration are increasingly recognized as key prognostic factors in myeloma and the achievement of a minimal residual disease (MRD)–negative CR predicts longer PFS and OS.35 Currently, the highest rates of MRD negativity are seen in early autologous HCT recipients.36,37 Moreover, the feasibility of autologous HCT at relapse could be negatively impacted by a decline in patient performance status or the development of comorbid illnesses. The results of ongoing studies of early versus delayed transplantation (e.g., NCT01208662 and NCT01208766) will eventually assist decision making in this setting.
Role of Autologous Transplantation in High-Risk Disease
Myeloma is a biologically diverse disease with clonal heterogeneity and genomic instability.38,39 The presence of t(4;14), del(17p), or high serum β2-microglobulin concentration predict inferior OS.40 Autologous HCT has provided suboptimal results in high-risk myeloma,41 questioning its benefit for these patients. Addition of bortezomib in induction, consolidation, and maintenance phases of therapy programs incorporating tandem HCT support the role of autologous HCT in high-risk myeloma.33,42,43
Unique Considerations for Hematopoietic Progenitor Cell Collection in Myeloma
In patients with myeloma who are autologous HCT candidates, it is important to avoid factors that impair HPC mobilization (e.g., MEL-based regimens, prolonged use of lenalidomide, and radiation therapy, to significant volume of bone marrow).44,45 While plerixafor has improved mobilization yields in difficult to mobilize patients with myeloma,46 intermediate-dose cyclophosphamide-based mobilization may be an equally effective and less expensive alternative.47 Given the increasing use of autologous HCT in the salvage setting after relapse from a prior autograft, it is prudent to collect ≥6 to 10 million CD34+ cells/kg at initial mobilization attempt.
Maintenance Therapy for Myeloma after Hematopoietic Cell Transplantation
Various consolidation and maintenance strategies have been evaluated to delay the inevitable post-HCT progression/relapse in myeloma. In general, novel agents have shown superior results as maintenance therapy. Thalidomide maintenance after tandem autologous HCT has been studied in several randomized studies.48–51 Lenalidomide maintenance was studied in the Cancer and Leukemia Group B 100104 and IFM 05-02 trials.52,53 Both studies demonstrated a superior PFS and EFS with lenalidomide maintenance, while Cancer and Leukemia Group B 100104 also demonstrated an OS benefit. A two- to three-fold increase in second primary malignancies was seen in both studies associated with the lenalidomide maintenance. Pretransplant bortezomib induction coupled with post-HCT bortezomib maintenance in the HOVON-65/GMMG-HD4 trial was shown to have superior PFS and OS.33 Based on these data, the risks/benefits and health-care costs of novel agent maintenance should be discussed with autologous HCT recipients.
Salvage Second or Third Transplants at Relapse
Salvage second autologous HCT is an option at relapse following a prior transplant. This is especially true for patients with available cryopreserved HPCs stored from their initial collection. A CIBMTR analysis showed that second salvage autologous HCT, while feasible, mostly benefits individuals relapsing ≥36 months from first autologous HCT.54 Following an initial tandem autograft, a (third) salvage autologous HCT at relapse while providing acceptable CR rates (29%) resulted in a disappointing 19% OS at 2 years.55
Future Directions in Autologous Hematopoietic Cell Transplantation for Myeloma
Recent advances in supportive care have made autologous HCT relatively safe and cost-effective. Current (2009–2011) CIBMTR data suggest a day 100 mortality of 1% in upfront autologous HCT for myeloma. Median 100-day cost for autologous HCT in the United States is approximately $100,000 US dollars, and largely represents the cost of hospitalization.56 Many institutions routinely perform autologous HCT entirely in the outpatient setting with excellent results.57,58 Robust quality systems, electronic chemotherapy ordering, and a support team of physicians, nurses, and pharmacists, etc., are essential to setting up an outpatient program.
Current directions being explored in HCT for myeloma include newer conditioning regimens and novel maintenance or consolidation strategies that aim to induce deeper responses. Based on the dose-response relationship of MEL in myeloma, escalated doses have been attempted with a maximum tolerated dose of 280 mg/m2 (limited by cardiac and gastrointestinal toxicity).59 Other approaches such as the combinations of busulfan and MEL60,61; busulfan and cyclophosphamide62; idarubicin and MEL63–65; thiotepa, busulfan, and cyclophosphamide64; bendamustine and MEL66; as well as bortezomib and MEL67 are without mature results.68 Approaches at delivering higher doses of radiation therapy to the marrow prior to autologous HCT without increasing overall toxicity are also being explored with helical tomotherapy or bone-seeking radioisotopes.69,70 Incorporation of modern imaging (positron emission tomography [PET] scans) and MRD detection in response assessment may enhance our ability to predict post-HCT outcomes and prognosis.71
AUTOLOGOUS HEMATOPOIETIC CELL TRANSPLANTATION FOR LYMPHOID MALIGNANCIES
Autologous HCT is standard for relapsed, aggressive non-Hodgkin lymphoma (NHL) and classical Hodgkin lymphoma (HL), and is curative in ~40% to 45% of patients.72–75 The role of HCT in indolent NHL and T-cell NHL is more controversial. The role of autologous HCT in lymphoma has recently been reappraised as conventional therapies have dramatically improved with the advent of monoclonal antibodies76,77 and radioimmunotherapy (RIT).78
Conventional and Novel Conditioning Regimens
Commonly used conditioning regimens for lymphomas include cyclophosphamide, etoposide, and carmustine; carmustine, etoposide, cytarabine, and melphalan (BEAM); carmustine, etoposide, cytarabine, and cyclophosphamide; and TBI-containing regimens.79 Limited retrospective data suggest lower TRM and superior outcomes following TBI-free conditioning.80 Several uncontrolled studies have reported efficacy and feasibility of combining rituximab with high-dose therapy,81,82 but convincing randomized evidence for this approach is lacking.
RIT with monoclonal antibody-radionuclide conjugates is effective in B-cell NHL. Incorporation of RIT into preautologous HCT conditioning has been studied, either alone or in combination with high-dose therapy.83–86 Two randomized trials have examined the latter approach. In the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0401 trial, iodine-131 tositumomab was prospectively compared against rituximab, both in combination with BEAM conditioning for relapsed diffuse large B-cell lymphoma (DLBCL).87 Another study compared BEAM plus yttrium-90 ibritumomab tiuxetan to BEAM alone in aggressive NHL.88 Neither study favored a RIT-based conditioning regimen.
Disease-Specific Indications for Hematopoietic Cell Transplantation in Lymphoid Malignancies
The indications of autologous HCT in lymphoid neoplasia are dependent on patient age, comorbidities, chemosensitivity, histologic subtype, and remission status at the time of HCT.
Hematopoietic Cell Transplantation for Follicular Lymphoma
Hematopoietic Cell Transplantation for Follicular Lymphoma: First Remission. The role of autologous HCT as consolidation for advanced-stage follicular lymphoma (FL) in first remission was examined in four randomized trials, including three trials conducted in the prerituximab era89–91 and one in rituximab era92 (Table 130.2). Autologous HCT provided a PFS benefit in three out of these four trials. OS, however, was not improved as this therapy was associated with a higher risk of second malignancies,91 including myelodysplastic syndrome/acute myeloid leukemia (AML).89,92 With the advent of RIT consolidation78 and rituximab maintenance93 or retreatment,94 the routine use of upfront HCT consolidation in FL is not recommended.
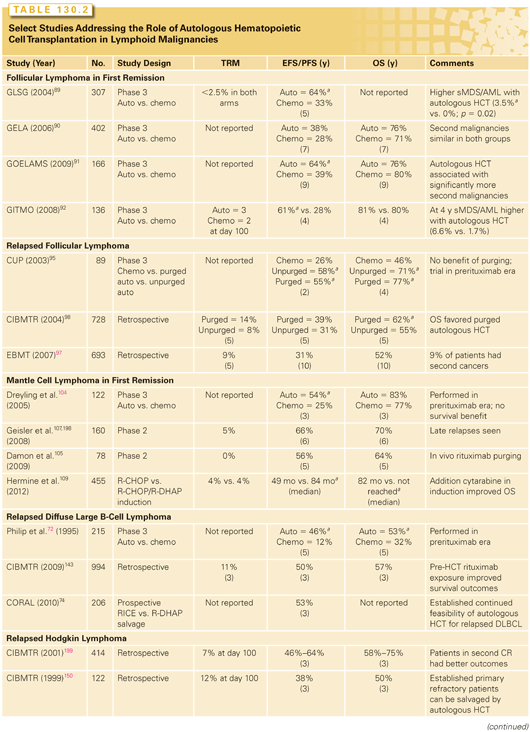
Hematopoietic Cell Transplantation for Follicular Lymphoma: Relapsed Disease. The role of autologous HCT in relapsed FL is controversial. In the prerituximab era, the CUP study compared salvage chemotherapy alone versus chemotherapy followed by either unpurged or purged HCT for relapsed FL; overall, the investigators reported a PFS and OS benefit with autologous HCT (see Table 130.2).95 More than a decade later, the superiority of autologous HCT over modern salvage chemoimmunotherapies is debated.96 Registry data from the European Blood and Marrow Transplant Group (EBMT)97 and CIBMTR show no plateau in relapse rates post-HCT in FL98 and a 5% to 15% risk of second malignancies97,99 (see Table 130.2). Autologous HCT for relapsed FL should be considered in the context of alternative treatment choices, patient age, remission status, comorbidities, and a small but definite risk of secondary cancers. Autologous HCT is best reserved for chemotherapy-sensitive, relapsed FL in those subjects who are not candidates for curative intent allogeneic transplantation.
Hematopoietic Cell Transplantation for Transformed Follicular Lymphoma. An EBMT report of 50 patients receiving autologous HCT for chemotherapy-sensitive transformed FL described 5-year PFS and OS of 30% and 51%, respectively.100 A prospective Norwegian study also reported 5-year PFS and OS of 32% and 47%, respectively.101 In the rituximab era, a Canadian cohort analysis suggested an OS benefit for patients undergoing HCT compared with those receiving chemoimmunotherapy alone.102,103 Autologous HCT is appropriate for transformed FL patients with nonbulky (nodal areas ≤3 cm), chemotherapy-sensitive disease.
Hematopoietic Cell Transplantation for Mantle Cell Lymphoma
The European MCL Network trial randomized patients with mantle cell lymphoma (MCL) after first-line chemotherapy to either upfront autologous HCT consolidation or interferon maintenance (see Table 130.2)104 and demonstrated a superior PFS but no OS benefit for HCT. Similar randomized trials in the rituximab-era after first-line chemoimmunotherapy are not available. Rituximab-era nonrandomized trials examining upfront HCT in patients with MCL have reported encouraging 5-year PFS and OS of ~60% and 70%, respectively105–110 (see Table 130.2). Despite the lack of randomized data, autologous HCT for MCL in first remission can be considered standard practice as retrospective data suggest that HCT after relapse may not be as effective.111,112 A CIBMTR analysis reported a 5-year OS of 61% for upfront HCT versus 44% for HCT performed in chemosensitive relapse.113 In patients with chemotherapy-sensitive, relapsed MCL, who are not candidates for allogeneic transplant, autologous HCT is reasonable; however, it is not recommended for therapy-refractory MCL.
Hematopoietic Cell Transplantation for Waldenström Macroglobulinemia
Prospective data are not available to recommend the use of autologous HCT for Waldenström macroglobulinemia (WM) in first remission. On the other hand, retrospective studies and expert opinion114 support the role of HCT in patients with relapsed/refractory WM.115–118 In an EBMT study of 158 patients with mostly chemotherapy-sensitive WM,119 5-year PFS and OS were 40% and 68%, respectively. Autologous HCT should be considered in patients with relapsed, chemotherapy-sensitive WM who already have received two or three different therapies.
Hematopoietic Cell Transplantation for Marginal Zone and Small Lymphocytic Lymphoma
Limited retrospective studies for HCT in marginal zone lymphoma120,121 indicate PFS rates similar to FL and frequent relapses after transplant. In patients with relapsed but chemotherapy-sensitive marginal zone lymphoma, autologous HCT could be considered. Data for autologous HCT for small lymphocytic lymphoma are scant. Extrapolating the data showing lack of a survival benefit for autologous HCT122–124 in chronic lymphocytic leukemia, HCT is not recommended in patients with small lymphocytic lymphoma.
Hematopoietic Cell Transplantation for Diffuse Large B-Cell Lymphoma
Hematopoietic Cell Transplantation for Diffuse Large B-Cell Lymphoma: In First Remission for High-Risk Disease? Studies using autologous HCT as consolidation after first-line therapies in aggressive (mostly DLBCL) NHL in the prerituximab era have reported contradictory findings. While the majority reported no benefit125–131 or (in one case) inferior outcomes,132 a handful of trials reported PFS and/or OS benefit with HCT either in general133–135 or in patients with high or intermediate-high International Prognostic Index (IPI).133 Studies from the rituximab-era restricted to higher-risk patients have also reported contradictory outcomes: two reports showed no benefit,136,137 whereas one intergroup US study suggested improved PFS and OS with autologous HCT consolidation high-risk IPI DLBC.138 Considering these discordant data and the high cure rates of DLBCL,139 use of upfront autologous HCT for this histology is not generally recommended.
Hematopoietic Cell Transplantation for Diffuse Large B-Cell Lymphoma: In Relapsed Disease. The role of autologous HCT in relapsed DLBCL is well-defined. The PARMA trial (see Table 130.2)72 established that salvage chemotherapy plus HCT consolidation provided an OS benefit in subjects with relapsed, chemotherapy-sensitive disease. Several registry140–143 and prospective studies in the rituximab era74 (see Table 130.2) have reproduced these results. Autologous HCT is thus the standard-of-care for relapsed/chemotherapy sensitive DLBCL.
Hematopoietic Cell Transplantation for Burkitt Lymphoma
Historically, brief induction therapies followed by upfront autologous HCT provided durable disease control in Burkitt lymphoma (5-year OS and PFS of 81% and 73%, respectively).144 However, the contemporary intense chemoimmunotherapy regimens are curative for majority of patients,145,146 and autologous HCT in first CR is not necessary. Fortunately, the grim prognosis for the patient with relapsed/refractory Burkitt lymphoma is uncommon. Retrospective studies147,148 describe 3- to 5-year OS and PFS rates of ~30% and 25%, respectively, after autologous HCT in chemotherapy-sensitive relapses.
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
Hematopoietic Cell Transplantation for Relapsed Hodgkin Lymphoma. Autologous HCT is potentially curative for relapsed HL. The British National Lymphoma Investigation study compared autologous HCT against salvage chemotherapy alone in relapsed HL149 and reported a PFS benefit in the high-dose therapy arm (53% versus 10%; p = 0.02). In another randomized trial, two cycles of chemotherapy were followed by two additional chemotherapy cycles versus autologous HCT.75 In chemotherapy-sensitive patients, freedom from treatment failure was significantly better with high-dose therapy. An OS benefit was not shown in either of these studies, most likely because those subjects assigned to the chemotherapy arm could still be salvaged by later HCT. Autologous HCT is the standard-of-care in chemotherapy-sensitive first relapse of HL.
Hematopoietic Cell Transplantation for Primary Refractory Hodgkin Lymphoma. Primary refractory (<CR after first-line therapy) HL has a poor prognosis. Lazarus and coworkers150 showed that autologous HCT can cure about a third of patients with primary refractory HL (see Table 130.2). Chemotherapy-refractory disease at autologous HCT is a poor prognostic factor (5-year PFS of <20%).151 While HCT is curative for HL, the 10-year late relapse rate (after the first 2 years) was 17% accounting for 25% of late mortality in a registry analysis.152 Autologous HCT is standard-of-care for relapsed HL, including those with primary refractory disease.
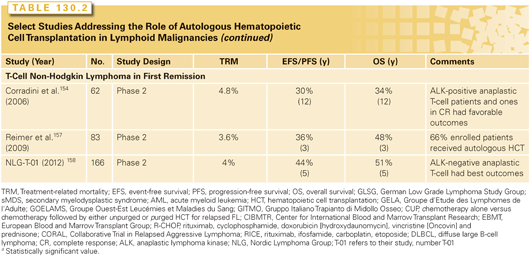
T-Cell Lymphomas
Relapsed T-cell NHLs have poor prognosis with chemotherapy alone,153 and autologous HCT has been explored as initial therapy or at the time of tumor progression. Phase 2 studies (with heterogeneous subtypes) examining upfront HCT in T-cell NHL (see Table 130.2)154–158 suggest a 3- to 4-year PFS of ~30% to 50%. Although randomized data are not available, considering the poor outcomes with conventional therapies, it is appropriate to consider upfront autologous HCT consolidation in T-cell NHL. Because of its excellent prognosis with standard chemotherapies, autologous HCT in first CR is not recommended for anaplastic lymphoma kinase–positive anaplastic large cell lymphoma.159 Relatively encouraging outcomes for a highly select group of patients with relapsed T-cell NHL with sensitive disease also have been shown with 3- to 5-year PFS and OS rates of approximately 15% to 30% and 30% to 45%, respectively.160–162 Autologous HCT is also reasonable in those patients with relapsed/chemotherapy-sensitive T-cell NHL not deemed candidates for allogeneic HCT.
Unique Considerations for Hematopoietic Cell Transplantation Mobilization in Lymphomas
While many patients with lymphoma can successfully mobilize HPCs with “cytokine only” strategies, mobilization failure remains a concern in patients with prior radiotherapy, heavily pretreated disease; exposure to hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone), fludarabine-containing regimens, or RIT; advanced age; and bone marrow involvement.114,163–166 In such cases, consideration should be given to chemotherapy- or plerixafor-based mobilization strategies.167
Tumor Cell Contamination in Autograft
Relapse after autologous HCT derives usually from proliferation of a chemotherapy-resistant clone of lymphoma cells surviving the high-dose therapy, or rarely from re-infusion of an autograft contaminated by tumor cells. Studies of syngeneic HCT suggest that a “tumor-free” graft may prevent/delay relapse.168 Ex vivo purging (by monoclonal antibodies, CD34+ cell selection, etc.)169,170 or in vivo purging (e.g., rituximab therapy during mobilization)171,172 of autografts appear to reduce or eliminate the risk of tumor-cell re-infusion at HCT. Reduction of tumor cell re-infusion, however, may not lead to improved outcomes. The CUP trial in relapsed FL95
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








