Abstract
Staging of breast cancer is critical for making treatment decisions as well as for prognosis. Staging systems have evolved over time, and most providers caring for patients with breast cancer use American Joint Commission on Cancer staging. This chapter also provides a review of multicentric and multifocal characteristics of breast cancer.
Keywords
breast cancer, breast neoplasm, staging, multicentric, multifocal
Staging: Past, Present, and Future
Staging plays an integral role in the management of breast cancer. Classifying patients by stage places them in prognostic groupings based on the best current available evidence gained from observation studies or clinical trials. The role of the surgical oncologist both past and present has been to acquire the pathologic tissues required to accurately diagnose, measure, and determine nodal staging of patients’ tumors. The major disciplines of surgery, medical oncology, and radiation oncology use current breast cancer staging classifications for determining the extent of disease, predicting overall survival, and providing guidance for therapy. Furthermore, epidemiologists and public health researchers rely on uniform breast cancer staging methods to evaluate trends in breast cancer incidence, screening programs, treatment outcomes, and risk factors worldwide. Finally, staging also plays an integral part in advances in breast cancer research and in the application of basic science to clinical science (translational research).
In the past, the cancer staging system was quite simplistic. Neoplasms were staged on the basis of clinical evaluation alone as operable or inoperable and classified as local, regional, or metastatic. However, there were limitations in accurately predicting the outcome in patients, and therefore the importance of deriving a more sophisticated staging system had been realized. Clinical staging is largely based on simple physical measurements such as size of the tumor or extent of anatomic spread of the disease to regional lymph nodes and distant organ sites at the time of diagnosis. These measurements are static and permit only limited evaluation of an ever-changing, heterogenous neoplasm that often has been in existence for years before the initiation of the staging process. Furthermore, advancements in breast cancer research and the development of new therapies have led to more treatment options (e.g., systemic chemotherapy, hormonal and other novel biologic therapies); however, the impact of treatment on long-term prognosis is not incorporated. Even today, the classification schemas for staging patients are limited by the inability to accurately assess the biological behavior of tumors and only minimal levels of molecular data are available.
Current staging schematics use a combination of clinical and pathologic factors, which are both useful in providing prognostic information. Clinical parameters historically have been used to predict survival because of the simplicity in obtaining the data. The clinical system may serve to guide initial therapy based on all available preoperative data that include history, physical and laboratory examinations, and biopsy material. However, tumors are now detected at very early stages, before they become clinically evident; as a result, histopathologic staging based on the primary tumor and local/regional lymph nodes has proved to be more accurate in predicting survival. The pathologic system, because of its ability to precisely define the histology and the extent of disease, is more accurate for grouping of patients with similar prognoses and for planning subsequent therapies.
Future staging systems will likely include new technologies and in-depth molecular, genetic, and pathologic analysis of the tissue specimens. The introduction of the sentinel lymph node (SLN) technique has allowed more accurate staging with much less morbidity to the patient compared with a complete axillary node dissection. With improvements in the sensitivity of detection methods, including histopathologic assays and molecular techniques, microscopic and submicroscopic tumor metastases can be detected. The recent expansion of genetic knowledge associated with the Human Genome Project has provided a large number of molecular tools that may prove valuable in the evaluation of tumor progression and overall survival. Molecular staging using reverse transcriptase polymerase chain reaction (RT-PCR), microarray analysis, and proteomics to identify unique gene and protein expression profiles may allow the detection of occult cancer before it becomes clinically evident and may provide more accurate information to help determine prognosis and survival with minimal morbidity to the patient. Finally, more accurate molecular staging may allow ablative rather than extirpative procedures and molecular-directed systemic therapies to be used. The increasing use of molecular staging and assessment of tumor response could potentially affect future staging systems by including biological markers to pretreatment clinical stage and posttreatment pathologic staging.
Clinical, Pathologic, and Biological Markers and Factors in Determining Prognosis
Central to any staging system are identifiable objective tumor and host characteristics that are prognostic of tumor progression. A number of clinical and pathologic factors have been identified that may predict the long-term outcome in patients with breast cancer. Generally accepted prognostic factors include age, tumor size, lymph node status, histologic tumor type and grade, mitotic rate, hormone receptor status, and human epidermal growth factor receptor 2 (HER2/neu) status. A number of other biological factors have also been studied and provide information regarding the potential for aggressive behavior of tumors ( Table 37.1 ).
| Clinical Histopathologic Factors | Biologic Factors |
|---|---|
| Age | Angiogenesis (VEGF) |
| Tumor size | Proliferation (MIB-1/Ki67/mitotic index) |
| Tumor location | Growth factor receptor (EGFR/HER2/neu) |
| Tumor histology | Cell cycle regulators ( p53/c-myc/ cyclins) |
| Tumor grade | Proteases (uPA/PAI-1/cathepsin D) |
| Hormone receptor status | Bone marrow micrometastasis |
| Vascular invasion | Circulating tumor cells |
| Lymph node status | Molecular subtype |
| Distant metastasis | Multiparameter gene expression analysis (MammaPrint/Oncotype Dx) |
Prognostic factors are important for forecasting outcomes in individual patients and can be used to help refine treatment choices. A prognostic factor is capable of providing information on clinical outcome at the time of diagnosis, independent of therapy. Such factors are usually indicators of growth, invasion, and metastatic potential. Nodal status is the most important parameter used to define risk category in early breast cancer and is considered a “pure” prognostic factor; that is, nodal status does not affect response to systemic therapy. On the other hand, a predictive factor is capable of providing information on the likelihood of response to a given therapeutic modality. Such markers are either within the target of the treatment or serve as modulators related to expression and/or function of the target. Estrogen receptor status is a predictive factor because it indicates the likelihood of response to endocrine therapy, but its role as an independent prognostic factor is controversial. In contrast, expression of the HER2/neu oncogenes may not only be an important prognostic factor in at least some subsets of patients with breast cancer; it may also provide some indication of the likelihood of response to certain chemotherapeutic agents. Therefore the HER2/neu oncogene has a dual role as prognostic and predictive factor.
Traditionally the dogma has been that prognostic factors help physicians determine which patients with breast cancer need adjuvant therapy, whereas predictive factors indicate which adjuvant therapy is most appropriate. The most important benefit of prognostic classification may be to help physicians identify patients in whom adjuvant therapy could be avoided, thus preventing treatment-related side effects. In theory, the identification, validation, and application of suitable predictive and prognostic factors helps ensure that only those patients likely to benefit will receive a given treatment.
Clinical Factors
Clinical staging is based on physical examination and information gathered from various imaging modalities. A thorough physical examination should consist of inspection and palpation of all of the breast tissue, the skin overlying the breasts, and the regional lymph nodes (axillary, infraclavicular, supraclavicular, and cervical). Important characteristics to note are the tumor size, whether there is extension into the chest wall, involvement of the overlying skin (e.g., erythema, induration, or edema), and presence of lymphadenopathy. Mobility of the involved regional lymph nodes is also important to note as a prognosticating indicator, with fixed nodes with extracapsular extension having a worse prognosis.
Imaging modalities such as mammogram, ultrasound, and contrast-enhanced magnetic resonance imaging (MRI) are available and useful as an adjuvant to the physical examination. The US Food and Drug Administration first approved the use of digital mammography in 2000 and further approved three-dimensional tomosynthesis in 2011. Although the data are still somewhat limited regarding tomosynthesis, a recent retrospective analysis performed by Friedewald and coworkers that included almost 460,000 patients demonstrated its use was associated with an increase in the overall cancer detection rate with concurrent decrease in the recall rate.
In regard to MRI usage, there is sufficient evidence supporting use of MRI for screening purposes in women with a known genetic mutation as well as women with a lifetime risk of developing breast cancer greater than 20% ; however, its use in women with a known breast malignancy is more controversial. MRI is being used with increasing frequency in an effort to assess extent of disease in the affected breast, evaluate for presence of multifocal and multicentric disease, and detection of cancer in the contralateral breast. Opponents of this trend argue that the increased sensitivity of MRI leads to more false-positive results, further necessitating unnecessary biopsies and larger surgeries. In a meta-analysis performed by Houssami and colleagues, additional disease was detected on MRI in the affected breast in 16% of cases with only a 66% positive predictive value. Likewise, the Comparative Effectiveness of MR Imaging in Breast Cancer (COMICE) trial is a prospective randomized study that was designed to investigate the clinical efficacy of preoperative MRI in women with breast cancer. When comparing MRI findings with histopathology, they noted 38% of patients in whom mastectomy was felt to be necessary based on MRI findings would have been candidates for breast conservation based on histology.
Primary Tumor Characteristics
Tumor Size
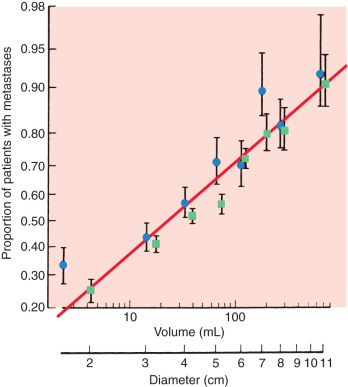
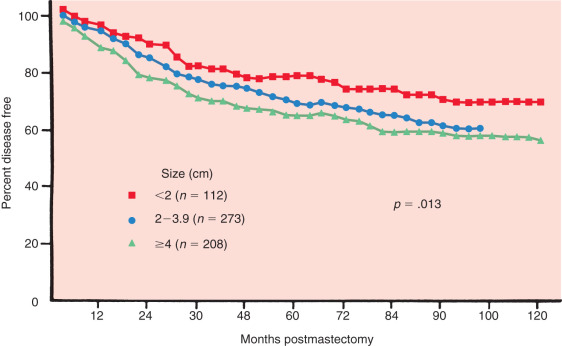
Tumor size is one of the most important prognostic markers of invasive breast cancer. It is defined as the maximal size of the invasive component of the primary tumor on pathologic examination. Clinical evaluation of tumor size has been included in many staging systems as an independent predictor of survival. The potential for metastasis increases in a linear relationship with the size of the primary tumor ( Fig. 37.1 ). Furthermore, there is a distinct relationship between increasing tumor size and the probability of axillary nodal metastasis. These investigators demonstrated that tumors must reach 3.1 to 4 cm in diameter to generate axillary metastasis in 50% of patients ( Table 37.2 ). Fisher has likewise shown that tumor size correlates with disease-free survival at 10 years, even when controlled for nodal metastases ( Fig. 37.2 ). Tumor size is particularly useful as a prognostic tool in patients with no involvement of regional lymph nodes and may alter adjuvant treatment options. For instance, patients with small tumors (<1 cm) who have lymph node–negative disease have an excellent overall prognosis. A study from the Surveillance, Epidemiology, and End Results (SEER) Program demonstrated that the 10-year breast cancer–specific mortality in this group of patients is only 4%, whereas their overall mortality is 24%. Thus, patients with small tumors with lymph node–negative disease have a fivefold higher risk of dying from causes other than breast cancer. It is also true, however, that patients with small tumors can have metastasis to the axillary lymph nodes, and conversely, more than one-third of patients with tumors greater than 6 cm in palpable diameter have negative lymph nodes, thus demonstrating the limited predictiveness of tumor size alone.
| Tumor Diameter (cm) | No. of Patients | Axillary Node Positive (%) |
|---|---|---|
| 0.1–0.5 | 147 | 28.6 |
| 0.6–1 | 960 | 24.7 |
| 1.1–2 | 4044 | 34.1 |
| 2.1–3 | 3546 | 42.1 |
| 3.1–4 | 1917 | 50.1 |
| 4.1–5 | 1135 | 56.5 |
| >5 | 1232 | 64.5 |
Tumor Location
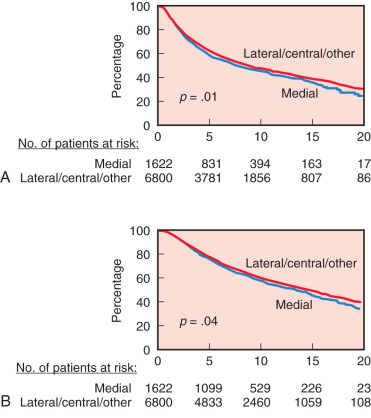
Tumor site is generally not included as a prognostic factor to identify patients at higher risk of relapse. However, tumor location has a significant prognostic utility, especially for axillary lymph node–negative patients. Several investigators from single institutions and data obtained from SEER have found that medial and central locations of tumor are associated with a 29% to 46% increase risk of developing systemic relapse and 20% to 46% increase risk of breast cancer–related death compared with the lateral location. A study from the International Breast Cancer Study Group that included more than 8000 patients confirmed these results but also demonstrated that the risk of relapse for patients with medial tumors was largest for the lymph node–negative patients and for patients with tumors larger than 2 cm ( Fig. 37.3 ). Furthermore, a recent study demonstrated that lower inner quadrant tumors have the highest risk of mortality compared with other locations in women with early lymph node–negative breast cancer. A proposed mechanism for the increased risk of metastases and death from breast cancer from medial tumors is occult involvement of internal mammary nodes (IMNs) that are not systematically treated with either surgery or radiation therapy. In fact, there is growing evidence that the lower inner quadrant drains more often to the IMNs than the other quadrants.
Tumor Histology
The great majority of breast carcinomas are adenocarcinomas that derive from the mammary glandular epithelial cells, most commonly cells from terminal ductal lobular units. Breast cancer is a heterogenous disease with up to 21 histologic subtypes. The most common type was classified as invasive ductal carcinoma, not otherwise specified (NOS); however, the nomenclature was recently changed by the World Health Organization (WHO) in 2012 to invasive carcinoma of no special type (NST). Approximately 25% of invasive breast cancers are classified as special types of breast cancer and show distinctive growth patterns, cytologic features, clinical presentation, and behavior. Next to inflammatory carcinoma, which is the most aggressive form of primary breast cancer with a 5-year survival rate of approximately 50%, pleomorphic lobular, metaplastic, and micropapillary carcinomas carry a poor prognosis. Tubular, cribriform, mucinous, papillary, medullary, and adenoid cystic subtypes have more favorable prognoses.
Tumor Grade
Histologic grading is a strong predictor of overall and disease-free survival in patients with invasive breast cancer; it is felt to be equivalent to lymph node status based on a number of independent studies. Nuclear grading is the cytologic assessment of tumor cells and it is applied to all invasive and in situ carcinoma of the breast. The WHO endorses a histologic grading system based on criteria established by Bloom and Richardson and Elston and Ellis, known as the Nottingham Grading System. This grading system incorporates three cytoplasmic and nuclear characteristics including extent of nuclear pleomorphism, degree of tubule formation, and number of mitotic features. Hensen and colleagues performed a SEER review of 22,616 cases of breast cancer and calculated 5-year survival rates based on grade alone. Survival at 5 years was estimated to be 93% for grade 1, 82% for grade 2, and 65% for grade 3. In addition, when evaluating grade and stage together, they noted that women with stage II disease and grade 1 tumors had the same survival rates as women with stage I disease and grade 3 tumors, suggesting that incorporating grade into current staging systems could help more accurately predict outcome.
Similarly, Fisher and colleagues examined histologic grade in relationship to 5-year treatment failures and found that there is a significant correlation between these two factors in patients with absent nodal metastases or with four or more positive lymph nodes. Furthermore poor histologic grade is a significant predictor of pathologic complete response (26%) compared with good histologic grade (14%) in the setting of neoadjuvant chemotherapy.
Histopathologic Features of Tumor
Fisher and associates examined the relationship of pathologic and clinical characteristics to 5-year survival in 1000 patients treated with radical mastectomy. Although they found that pathologic nodal status was the most dominant influence on treatment failure rates and corresponding survival, they also identified a number of other important predictors of short-term (<24 months) treatment failure. Characteristics such as perineural invasion, absence of sinus histiocytosis, presence of glycogen, and skin involvement correlated with poor long-term survival. Tumor necrosis was also associated with a poor clinical outcome. However, independent prognostic significance of this histologic feature is not established in the literature. Furthermore, quantification of necrosis has not been standardized.
Other histologic characteristics have also been found to bear prognostic information. These include lymphovascular invasion, elastosis, glycogen staining, and the presence or absence of numerous host inflammatory responses. Evaluation of lymphovascular invasion is particularly important in small lesions without nodal involvement because it can identify patients with high risk of recurrence. In addition, it has also been found to have a strong association with breast cancer–specific survival and distant disease free survival. On the contrary, presence of tumor-infiltrating lymphocytes has been associated with improved overall survival and distant disease-free survival, especially in triple-negative breast cancers.
Estrogen and Progesterone Receptors
Both estrogen receptors (ER) and progesterone receptors (PR) are essential prognostic indicators in patients with breast cancer, and more importantly, they are highly predictive of benefit from endocrine therapy in both the adjuvant and metastatic settings. Knight and colleagues and Osborne and McGuire have shown that ER status affects survival, independent of axillary nodal status with ER positivity generally correlating with a better prognosis. Similarly, another study has demonstrated longer survivorship for PR-positive patients than for PR-negative patients. Receptor status has also been shown to be predictive of response in patients receiving chemotherapy. In the neoadjuvant setting, several studies have confirmed that ER-negative tumors are more likely to decrease in size in response to chemotherapy. However, although ER-negative tumors are more likely to achieve pathologic complete response with neoadjuvant chemotherapy, affected patients have less favorable 5-year overall and progression-free survival rates compared with those with ER-positive disease. One proposed explanation for this finding was the beneficial effect of postoperative hormonal therapy available to patients with ER-positive tumors, which could supersede the significance of achieving pathologic complete response in this setting.
HER2/neu Expression
HER2/neu-positive phenotype accounts for about 15% of patients. Amplification or overexpression of HER2/neu has been found to portend a poor prognosis, correlating with other factors associated with poor prognosis such as tumor grade, size, nodal status, and hormone receptor status. Furthermore, it is also considered a predictive factor in that it predicts response to targeted therapy.
Tumor Growth Rate and Proliferation
Anatomic methods for staging breast cancer have withstood the test of time and remain the gold standard; however, these methods often provide only a static or instantaneous view of what is actually occurring at the molecular and cellular levels. Attempts at measuring dynamic characteristics (clinical and pathologic) have also been predictive of tumor progression. Clinical measurements of flux of tumor volume over time and pathologic determinations of cell kinetics have both been useful. Most of breast cancer prognostic factors are directly or indirectly related to proliferation, and increased proliferation correlates strongly with poor prognosis, irrespective of the methodology used to assess proliferation. Measurements of tumor doubling time, although not clinically practical, have been shown to correlate with prognosis. Multiple studies have demonstrated a wide range of doubling times, not only between patients but also within the same patient over time. This heterogeneity is typical of breast cancer. Evaluation of tumor cell kinetics through growth fraction estimates has been used for the assessment of proliferation rate and its relation to prognosis. The growth fraction can be assessed by immunohistochemistry (IHC) of proliferation-associated antigens, such as ki67, cyclin A, cyclin D, cyclin E, p27, p21, thymidine kinase (TK), topoisomerase IIα, proliferating cell nuclear antigen (PCNA), geminin, or minichromosome maintenance (McM) proteins. Of these, ki67 has extensively been studied alone or with MIB1, which is an antibody that reacts against ki67. There is a good correlation between ki67 and MIB1 staining, and a pronounced decrease in the ki67/MIB1 labeling index is associated with a good response to preoperative treatment with chemotherapy or tamoxifen. In a recent review by Luporsi and coworkers, ki67 was found to be significantly associated with disease-free survival with seven randomized controlled trials and two meta-analyses in multivariate analysis. In addition, in the neoadjuvant setting, an elevated ki67 was associated with a higher likelihood of complete pathologic response.
Flow cytometry has recently been used to estimate cellular kinetics and to detect the presence of aneuploidy. Flow cytometry permits rapid, single-cell analysis of DNA content per cell, enabling the determination of the fraction of cells within the S phase and the ploidy levels within tumor cell proliferation. However, despite extensive literature on flow cytometry, the results obtained by this method are still not widely used in everyday practice, because there is high intratumor heterogeneity of the S-phase fraction and multiple methodologies to determine S-phase fraction by flow cytometry. In general, a high S-phase fraction is associated with a higher risk of metastatic recurrence in lymph node–negative patients. Furthermore, it has been suggested that flow cytometry also influences the response to chemotherapy in neoadjuvant, adjuvant, and metastatic settings.
Other methods used to assess the growth fraction are the incorporation techniques with tritium-labeled thymidine (3H-TdR) and bromodeoxyuridine (BrdU), which theoretically provide the gold standard of cellular proliferation. These methods are more sensitive than standard histologic tests that enumerate the relative number of mitoses and have demonstrated some promise in their ability to identify aggressive, rapidly growing tumors. With tritiated thymidine, tumor cells are sampled from the specimen and then incubated with 3H-TdR, which is taken up by cells in the DNA synthetic or S phase. Measuring 3H-TdR uptake then permits the estimation of the percentage of cells in the S phase (thymidine labeling index). Many studies have shown that a high thymidine labeling index is associated with poor prognosis in lymph node–positive and –negative patients with breast cancer, and patients with a high index benefit from adjuvant chemotherapy. However, incorporation techniques can be tedious and time-consuming, and they are impractical for routine use; this hampers their worldwide application, despite the very good prognostic value of the thymidine labeling index. This index does, however, correlate with histologic characteristics such as tumor grade and may be important in the staging of lymph node–negative patients.
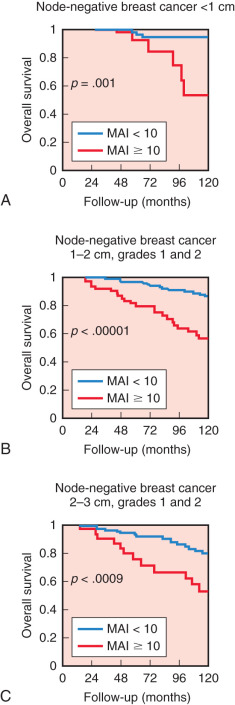
One the most powerful, practical, and well-reproduced indicators of proliferation and prognosis is the mitotic activity index (MAI). As mentioned previously, MAI is part of the histologic grade system and represents by far the most important contributor to the prognostic value of histologic grade. The proliferation factor MAI is one of the strongest prognostic factors in patients younger than 71 years with lymph node–negative invasive breast cancer, without adjuvant systemic treatment and with long-term follow-up. Moreover, patients with rapidly proliferating tumors significantly benefit from adjuvant systemic therapy, in contrast to those with low proliferation. Recently, Baak and colleagues demonstrated that among women with small, low-grade, lymph node–negative invasive breast cancer who usually do not receive systemic therapy, MAI (≥10/1.6 mm 2 ) was able to identify accurately those patients at high risk of distant metastatic disease or death. The 10-year overall survival rates for MAI less than 10 versus 10 or higher in women with tumor diameter less than 1 cm were 94% and 67%, respectively ( Fig. 37.4 ). The authors concluded that these high-risk patients identified by MAI should be considered for adjuvant systemic therapy.
Biological Markers
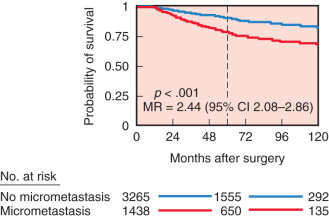
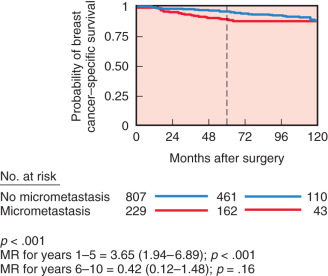
With the development of monoclonal antibody technology, antibodies specific for breast cancer have been developed. These antibodies can be used to detect microscopic disease not easily seen on routine histopathology by using immunoperoxidase technology and radioimmunoassay. They may be useful in accurately staging the anatomic extent of axillary disease and distant disease such as tumor infiltration of bone marrow. The presence of isolated tumor cells in the bone marrow of women with early-stage breast cancer may be an independent marker of disease recurrence and shortened survival. However, controversy exists regarding the independent influence of occult metastatic cells on prognosis. Braun and colleagues performed a pooled analysis involving more than 4700 patients from several prospective studies and revealed that bone marrow micrometastases did predict an independent poor outcome ( Fig. 37.5 ). Compared with women without bone marrow micrometastases, patients with bone marrow disease had larger tumors, tumors with a higher histologic grade, more frequent lymph node metastases, and more hormone receptor–negative tumors. Furthermore, in a subgroup analysis of low-risk patients with tumors less than 2 cm and without lymph node metastases who did not receive chemotherapy, the difference in distant disease-free survival between those patients who had micrometastases versus those who did not was very small ( Fig. 37.6 ). These data suggest that the presence of bone marrow micrometastases may often reflect other prognostic factors already discerned from the primary tumor characteristics and lymph node status. The American College of Surgeons Oncology Group (ACOSOG) Z10 study is a prospective observational study whose primary objective was to investigate the association between survival and disease-free survival in women with early-stage breast cancer (T 1–2 ) and detected metastases within SLNs and bone marrow specimens using IHC. Of the specimens noted to be negative on hematoxylin and eosin (H&E) stain that were further evaluated with IHC, 10.5% of SLNs and 3% of bone marrow aspirates were found to be positive for tumor. Importantly, no difference in overall survival or disease-free survival was seen in women with H&E-negative, IHC-positive SLNs. However, there was a significant association between decreased overall survival and occult bone marrow metastases with no significant difference in disease-free survival. The authors concluded that although presence of occult bone marrow metastases may help identify high-risk women, the incidence was too low to recommend routine bone marrow aspiration in women with early-stage breast cancer.
Similar to detection of disseminated tumor cells in bone marrow, novel methods that measure circulating tumor cells (CTCs) in the peripheral blood have also been developed. Currently, there is only one US Food and Drug Administration–approved test, the CellSearch assay (Veridex, Warren, NJ). This assay is based on the enumeration of epithelial cells, which are separated from the blood by antibody-coated magnetic beads and identified with the use of fluorescently labeled antibodies against cytokeratin and with fluorescent nuclear stain. Several studies have confirmed that the presence of CTCs is associated with a poor outcome in patients with metastatic disease. Cristofanilli and associates measured CTCs before systemic treatment in patients with metastatic disease and found that levels of CTCs equal to or higher than 5 per mL of whole blood were the most significant predictors of progression-free and overall survival. Furthermore, these CTC levels after the first course of hormone therapy or chemotherapy predicted no treatment response. A recent Southwestern Oncology Group (SWOG) study further confirmed the poor prognostic significance of CTCs in metastatic breast cancer patients; however, they failed to find a survival benefit of early change to an alternate systemic therapy. Although measuring CTCs shows promise, it still faces a number of challenges in terms of identification and full understanding of clinical significance, and their use in diagnosis, treatment management, or staging is currently not recommended.
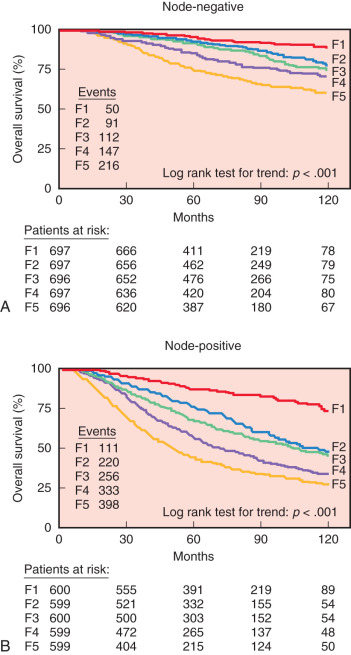
Tumor markers, such as CA15-3, CA27-29, and carcinoembryonic antigen (CEA), are not very sensitive or specific for breast cancer and are not recommended for screening, diagnosis, or staging. Cathepsin D, a lysosomal proteolytic enzyme with a critical role in protein catabolism and tissue remodeling, has been used as a marker of invasion and poor prognosis in breast cancer. However, the magnitude in predicting outcome is relatively small, and routine clinical use of this marker is also not recommended. Other novel biologic markers recently found to be associated with prognosis are the urokinase plasminogen activator (uPA) and the plasminogen activator inhibitor (PAI-1), which are part of the plasminogen activating system. Several studies have suggested that overexpression of uPA and/or PAI-1 are strongly associated with poor prognosis in lymph node–negative cancer, and when these two factors are combined, they are associated with a two- to eightfold higher risk of recurrence and death. Furthermore, Janicke and coworkers found in a prospective randomized trial that low or high levels of uPA and/or PAI-1 can help stratify who will benefit from adjuvant chemotherapy in patients with lymph node–negative breast cancer. In their study, for all women treated without systemic chemotherapy, the 3-year recurrence rate was significantly lower for those with low expression of uPA and PAI-1 (6.7% vs. 14.7%). The independent prognostic value of high levels of uPA and/or PAI-1 was confirmed in a pooled analysis involving 8377 breast cancer patients by the European Organization of Research and Treatment of Cancer-Receptor and Biomarker Group. In both lymph node–positive and lymph node–negative patients, higher levels of uPA and/or PAI-1 were independently associated with poor relapse-free and overall survival ( Fig. 37.7 ). Currently, uPA and/or PAI-1 measured by enzyme-linked immunosorbent assay has been recommended for the determination of prognosis in patients with newly diagnosed, lymph node–negative breast cancer by the American Society of Clinical Oncology. However, despite comprehensive validation, they are infrequently used clinically. Possible explanations include the large amount of tissue needed for the assay originally, the requirement of fresh or freshly frozen tissue, and the availability and convenience of other multiparameter assays, such as MammaPrint and Oncotype Dx.
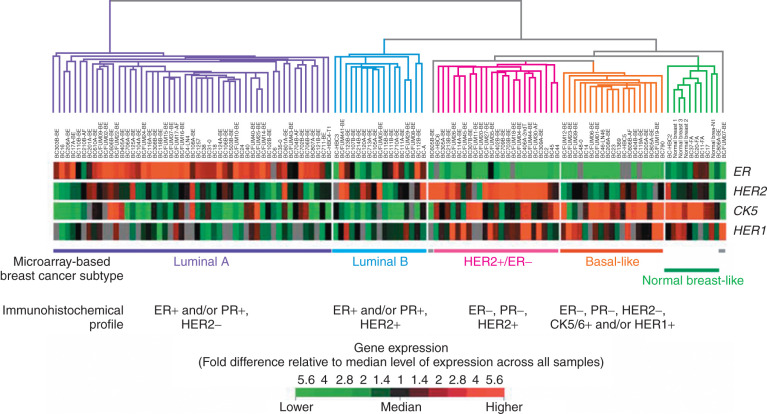
Oncogenes may be related to the complex phenomenon of human breast cancer initiation and progression through the process of gene amplification, mutation, chromosomal breakage, or insertion of retroviral promoters near oncogenes. Although studies of individual oncogenes and tumor suppressor genes may prove fruitful in identifying poor patient prognosis, simultaneous analysis of expression of multiple genes in individual tumors is a new approach to breast cancer classification and has shown promise in providing greater accuracy in predicting outcomes and in aiding selection of therapies for individual patients. Using RNA expression array technology, initially four and then after further refinement, five breast cancer molecular subtypes were identified and validated in both clinically and ethnically diverse patient populations. These five subtypes include basal-like, HER2/neu-overexpressing, luminal A, luminal B, and normal breastlike ( Fig. 37.8 ). These subtypes have been compared with clinical outcomes; luminal-type cancers tend to have the most favorable long-term survival, whereas the basal-like and HER2/neu-overexpressing are most sensitive to chemotherapy but have the worst prognosis overall ( Fig. 37.9 ).
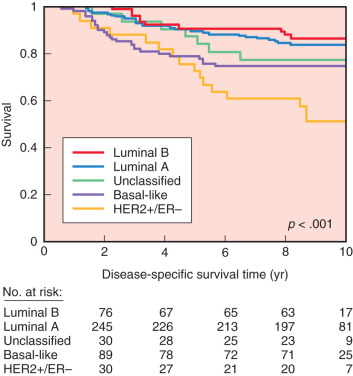
The true prognostic or predictive value of the molecular classes is unknown because there is a strong correlation between molecular class and conventional histopathologic prognostic factors. For example, Pusztai and colleagues found that all luminal-type cancers were ER positive, and 63% of these were also low or intermediate histologic grade. This contrasted with their finding that 95% of basal-like cancers were ER negative, and 91% were high histologic grade. Furthermore, the majority of basal-like cancers are characterized by the absence of ER receptor, PR receptor, and HER2/neu expression, and they are also associated with p53 gene mutations and a high proliferation rate. Because of these characteristics, basal-like cancer is usually referred to as triple-negative breast cancer in daily practice. However, although the terms tend to be used interchangeably, they are not completely synonymous, because a small proportion of basal-like cancers do express hormone receptors or HER2/neu . Several studies have confirmed that triple-negative breast cancers are characterized by an aggressive clinical history, an earlier age of onset, and BRCA1 -related breast cancer. Recently, Bauer and colleagues found that the relative survival for women with triple-negative breast cancer was poorer than for women with other types of breast cancer, with 77% of women surviving 5 years after diagnosis, compared with 93%. Finally, several studies have confirmed that African American women and Hispanic women were found to have a higher incidence of triple-negative phenotype. These recent results might explain biologic disparities in long-term survival of breast cancer associated with race.
Molecular profiling has also been used to stratify patients with early breast cancer into prognostic groups, and information gained by these methods may outperform standard clinical and pathologic prognostic features. Several multiparameter assays that do not overlap have been developed and include the Rotterdam signature, the Breast Cancer Gene Expression Ratio, MammaPrint, and Oncotype Dx, but only the last two are currently commercially available. For instance, MammaPrint (Agendia BV, Amsterdam, the Netherlands), is a 70-gene signature (largely consisting of genes regulating proliferation, invasion, metastasis, stromal integrity, and angiogenesis) used to stratify patients into a prognostic group using DNA microarrays originally in fresh frozen tissue. This was designed by comparing gene expression in lymph node–negative patients, younger than 53 years of age with T 1 or T 2 breast cancers who developed metastases within 5 years of diagnosis, compared with a matched group that did not develop metastasis. At 10 years, van de Vijver and colleagues demonstrated that both overall survival (95% vs. 55%) and distant metastasis-free survival (85% vs. 51%) were significantly greater in those classified as having a good prognosis according to their gene expression profile alone. The estimated hazard ratio for distant metastases by signature was 5.1 ( p < .001) and remained significant when adjusted for lymph node status. This signature has since been validated with an independent data set, can now be performed using formalin fixed paraffin embedded tissue, and is currently being utilized in prospective, randomized trial titled Microarray In Node Negative Disease May Avoid Chemotherapy (MINDACT). The hypothesis of the trial is that molecular profiling can lead to improved risk assessment thereby reducing the number of patients receiving adjuvant systemic therapy without compromising long-term outcome. Results of the pilot phase including the first 800 patients was published and showed that, compared with Adjuvant! Online, use of MammaPrint resulted in a potential 8.25% reduction in chemotherapy use. Results of long-term outcome are still pending.
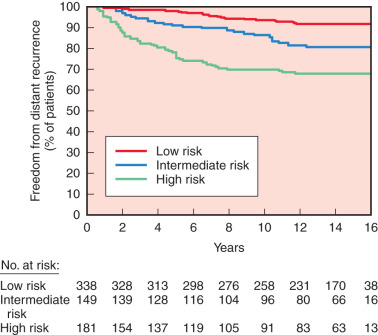
On the other hand, Oncotype Dx (Genomic Health Inc., Redwood City, CA) is a 21-gene real-time RT-PCR assay on formalin-fixed, paraffin-embedded tissue, with 15 cancer-related genes and six reference genes, that is currently being studied to help predict the chance of recurrence in patients with lymph node–negative, ER-positive breast cancer. The assay calculates a recurrence score, which stratifies patients into low, intermediate, and high risk of recurrence. Paik and colleagues evaluated this assay among women treated with tamoxifen in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial. Kaplan-Meier estimates of 10-year disease recurrence rate in the three groups were 6.8%, 14.3%, and 30.5% ( Fig. 37.10 ), respectively, and there was also a significant relationship between recurrence score and overall survival. Currently this assay is being studied in a clinical trial (Trial Assigning IndividuaLized Options for Treatment [TAILORx]), evaluating the role of chemotherapy in lymph node–negative patients. Patients deemed to be high risk are given chemotherapy and hormone therapy, patients in the intermediate group are randomized to hormonal therapy alone or combination chemotherapy plus hormonal therapy, and patients in the low-risk group are treated with hormone therapy alone. The initial results of the lowest risk group (scores of 0–10) were recently published. At 5 years the rate of freedom of recurrence from breast cancer at a distant site in these 1626 women assigned to receive endocrine therapy alone was 99.3% and the overall survival was 98.0%. As more results from this trial enrolling more than 10,000 women emerge, the utility of the Oncotype Dx will continue to evolve.
Lymph Node Status
In the late 19th century, Halsted described how breast cancer spreads systematically from the primary breast tissue to the ipsilateral axilla and is finally disseminated into the systemic circulation. The presence of axillary nodal metastasis was considered a poorer prognostic factor compared with improvement in survival with a more radical operation. However, this theory has been challenged over the years when it became evident that more extensive mastectomies proved to have survival benefit equal to that of less extensive, breast conserving operations. Furthermore, up to 30% of patients with lymph node–negative disease eventually develop recurrent metastatic disease. However, once the diagnosis of breast cancer has been established, the lymph node status remains the most powerful predictor for long-term survival. Over the years, lymph node staging has been refined to include the number of lymph nodes, and more recently, the amount of tumor burden within these nodes. Not only does the lymph node status give prognostic information, it is also important in making therapeutic decisions.
The clinical assessment of regional lymph nodes must include ipsilateral axillary nodes and interpectoral, or Rotter, nodes. Other regional nodes include the IMNs in the intercostal spaces along the sternum and supraclavicular nodes. For a lymph node to be considered supraclavicular, it must be located in a triangle bound by the clavicle at the base, the internal jugular vein medially, and omohyoid muscle and tendon laterally and superiorly. Physical examination, however, is notoriously inaccurate in preoperative assessment of the presence of lymph node metastasis. In fact, microscopic evidence of tumor can be demonstrated in one-third of patients in the absence of palpable axillary lymph nodes. Clinical examination has a false-positive rate for detection of axillary metastases ranging from 25% to 31%. False-negative rates range from 27% to 33%.
Axillary Nodal Disease
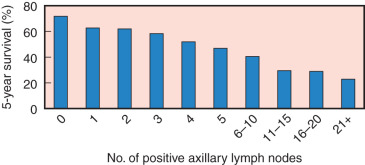
Results of a national survey by the American College of Surgeons (ACS) involving 20,547 women with breast cancer clearly demonstrated a strong linear association between the number of histologically involved axillary nodes and 5-year survival ( Fig. 37.11 ). Furthermore, in a 10-year clinical trial, Fisher, Fisher, and Redmond, reporting for the NSABP, found that patients with negative axillary lymph nodes had 5- and 10-year survival rates of 78% and 65%, respectively. Similarly, the number of positive nodes correlated with the 5- and 10-year treatment failures. No positive nodes was associated with a 20% treatment failure rate at 10 years, whereas more than four positive nodes was associated with a 71% treatment failure rate. Not only is axillary lymph node metastasis a time-dependent variable, but it also seems to be a marker for a more aggressive tumor phenotype. Jatoi and associates have shown that patients with four or more involved lymph nodes at the time of initial diagnosis have a significantly worse outcome after their first recurrence ( Fig. 37.12 ).

Although axillary nodal status is an important prognostic indicator, its therapeutic role is more to clear the axilla of gross disease. Historically, there was a push to perform extensive axillary dissections to obtain accurate prognostic information. Certainly, complete dissection of all three levels of lymph nodes (Patey dissection) not only provided the maximum amount of prognostic information but also cleared the axilla of gross disease and was thought to obviate the need for axillary irradiation. Before the advent of SLN techniques, several studies addressed the extent of axillary dissection and determined that, in certain cases, a level 1 dissection could be predictive of the actual involvement of the remaining nodal basin. Boova and colleagues examined the contents of 200 consecutive mastectomy and total axillary dissection specimens. The average number of lymph nodes recovered from each level was 14 at level 1, 11 at level 2, and 8 at level 3. Of the patients, 40% had axillary metastases, with half of these involving only level 1 ( Table 37.3 ). Interestingly, seven patients (3.5% of all patients and 8.7% of those with lymph node metastases) had positive nodes at level 2 and/or 3 without positive nodes at level 1 (skip metastasis). These authors concluded that level 1 dissections could be accurate predictors of the status of the entire axillary lymph node basin, assuming that an adequate number of lymph nodes has been sampled. Fisher and coworkers also suggested that dissection of levels 1 and 2 is more than adequate in most cases to accurately predict prognosis. The current standard of care for a complete axillary lymph node dissection includes both level 1 and level 2 lymph nodes with procurement of at least 10 lymph nodes.
| Nodal Status | No. of Patients (%) |
|---|---|
| All levels negative | 120 (60) |
| Level 1 positive | 39 (19.5) |
| Levels 1 and 2 positive | 19 (9.5) |
| Levels 1, 2, and 3 positive | 11 (5.5) |
| Levels 1 and 3 positive | 4 (2) |
| Level 2 positive a | 5 (2.5) |
| Level 3 positive a | 1 (0.5) |
| Levels 2 and 3 positive a | 1 (0.5) |
Sentinel Lymph Node Mapping
The introduction of SLN mapping technology has challenged the classic approach to breast cancer staging that involves routine axillary lymph node dissection. The SLN concept supports the notion that breast cancer cells spread in an orderly fashion via direct lymphatic communication from the primary tumor to the SLN. Therefore the SLN is most likely to be the first site of metastasis within the lymph node basin. Currently, SLN mapping for breast cancer has become the primary means for accurately assessing nodal metastasis. On average, one to three SLNs may be found within an axillary basin. A number of large, single, and multiinstitutional trials have now demonstrated the accurate predictability of the SLN technique in staging the axilla in breast cancer patients by using various injection techniques with technetium sulfur colloid, isosulfan blue, or a combination of both agents. The optimal technique for most is a combination of blue dye and technetium sulfur colloid. A SLN identification rate of more than 95% is the rule, with accuracy rates exceeding 95% and a false-negative rate of approximately 2%. A well-trained multidisciplinary team must be systematically assembled to ensure the success of the procedure. Long-term follow-up is needed to define the true false-negative rate—that is, to identify axillary recurrences in patients with breast cancer with negative SLNs and no complete axillary dissections. Initial studies suggest that this risk is less than 1% (0.2%–0.3%).
In addition, the accuracy of the SLN mapping technique is enhanced by the ability to improve on the histologic examination of the lymph node when 1 to 3 lymph nodes, as opposed to 15 to 20 lymph nodes, in a complete node dissection are submitted for a more detailed examination. Routine histologic examination with serial step sectioning and H&E staining is performed to identify tumor deposits within the lymph node. Furthermore, IHC, with cytokeratin assays, shows an increased rate of detection of occult micrometastatic disease, further improving the accuracy of the technique. Cote and colleagues noted that occult nodal metastases were detected in 7% of patients with H&E sections and in 20% with IHC. Other investigators have also shown the increase in detection of occult micrometastatic disease in patients who were determined to be lymph node–negative based on routine histologic methods. In a study by Schreiber and associates, up to 9.4% of the patients were upstaged with the addition of IHC and serial sectioning. However, to date the clinical significance of identifying micrometastatic and submicrometastatic disease as a prognostic variable is controversial. A population-based analysis by Chen and coworkers evaluated 209,720 patients with invasive breast cancer without distant metastases and no more than three axillary nodes using the SEER registries. Overall, patients with micrometastases no larger than 2 mm had a significant worse 5-year survival (86%) than patients without nodal metastases (90%) and better than patients with macrometastases in no more than three nodes (82%; Fig. 37.13 ). Cox and coworkers noted that 16% of patients with micrometastasis (>0.2 mm and <2 mm) and 9% of patients with isolated tumor cells or small-cell clusters not greater than 0.2 mm by IHC on SLN biopsy will have additional positive non-SLN if an axillary lymph node dissection is performed. As stated previously, ACOSOG Z10 showed that of the specimens noted to be negative on H&E stain that were further evaluated with IHC, 10.5% of SLNs were found to be positive for tumor with no difference in overall survival or disease-free survival compared with women with IHC negative SLNs.

Internal Mammary Nodal Disease
The status of the IMNs has been a point of controversy since the time of Halsted in the 1890s. Prospective randomized trials have failed to demonstrate a benefit in survival for women who had complete IMN dissections (extended radical mastectomy) while incurring significant operative morbidity. However, other studies have shown a prognostic significance of metastasis to the IMNs. Veronesi and associates reported 10-year overall survival rates according to IMN disease and/or axillary lymph node disease among patients who had extended radical mastectomy ( Fig. 37.14 ). The study clearly demonstrated a marked difference in overall survival between patients without any nodal disease (80%) and patients with only IMN disease and only axillary lymph node disease, with an overall survival of 53% and 55%, respectively. Furthermore, the presence of both IMN disease and axillary lymph node disease was associated with the worst outcome with an overall survival of 30%. Positive IMNs can be found in up to 10% of cases when axillary nodes are negative and in as many as 26% of cases when the primary tumor location is medial. Lymphatic mapping techniques may lead to the ability to identify IMNs with improved accuracy; however, there is still appreciable morbidity. The surgical management of IMNs remains controversial.

Supraclavicular Nodal Disease
Historically, supraclavicular lymph nodes involved with tumor indicated advanced disease and were associated with a poor prognosis. Positive supraclavicular lymph nodes were considered to have survival rates similar to those with distant spread rather than of regional nodal disease. More recent studies suggest that the outcomes of patients with these tumors are comparable to those of patients with locally advanced disease rather than distant metastatic disease.
Currently, routine scalene lymph node biopsies are not indicated. In the prechemotherapy era, scalene lymph node biopsy was performed to determine operability of breast cancer patients with advanced local disease but without evidence of distant metastases. It was noted by Papaioannou and Urban, however, that when scalene lymph nodes were pathologically positive, despite the use of radical mastectomy with adjuvant radiotherapy, no patients were disease free after 1 year, and more than 50% were dead within 3 years. These data once again suggested that metastasis to lymph node beds other than regional (axillary, clavicular, and internal mammary) suggests the presence of distant systemic disease.
Intramammary Nodal Disease
Intramammary lymph nodes (intraMLNs) have received little attention as potential prognostic indicators for patients with breast carcinoma and are largely overlooked by radiologists, pathologists, and surgeons. Recently interest for intraMLNs as a marker of disease severity has increased with the introduction of SLN biopsy and lymphatic mapping because detection of intraMLNs is likely to occur more frequently by preoperative lymphoscintigraphy and intraoperative gamma probe scanning of the breast. By definition, intraMLNs are surrounded by breast parenchyma, a feature that distinguishes them from low axillary lymph nodes. Histopathologic studies of breast specimens containing intraMLNs have revealed that these lymph nodes may be present in any quadrant of the breast and can yield a variety of pathologic findings, including metastatic carcinoma in the ipsilateral breast.
The prevalence of intraMLNs has been reported to range between 1% and 28%. Egan and McSweeney were the first to address the prognostic significance of intraMLN in breast cancer. They found that in stage I disease, positive intraMLNs were associated with a poor prognosis (10-year survival rate, 79%); however, intraMLNs were not found to be associated with poorer prognosis in stage II disease. Because of this, patients with breast carcinoma accompanied by intraMLN metastases are traditionally considered to have stage II disease, even in the absence of axillary lymph node involvement. Recently Shen and colleagues evaluated 130 patients with intraMLNs. IntraMLN metastases were found in 28% of all cases, and isolated intraMLN metastases were documented in 5% of all cases. The presence of intraMLN metastases was associated with poorer 5-year overall survival compared with non-intraMLN involvement (64% vs. 88%). Furthermore, intraMLN was found to be a significant independent predictor of survival in patients with axillary lymph node involvement as well as among those without axillary involvement. Similar results have reported that intraMLN metastases is a marker of disease severity and that the presence of metastatic disease in an intraMLN is associated with a high rate of axillary nodal involvement.
Pathologic Assessment of Lymph Nodes
Molecular staging using techniques such as RT-PCR is allowing the detection of one tumor cell in the background of a million. Adoption of these technologies has raised the likelihood of finding micrometastases. Furthermore, accurate molecular analysis of all or part of the SLN offers the potential to reduce false-negative findings significantly and overcome limitations that occur with standard methods of intraoperative SLN analysis. Currently, intraoperative SLN analysis methods such as imprint cytology (touch preparation) and frozen section suffer from poor and variable sensitivity and a lack of standardization. Compared with final pathology results, the reported sensitivity of frozen section SLN analysis varies from 58% to 87%. Imprint cytology has similar limitations in sensitivity. Recently, Backus and colleagues were able to identify an optimal two-gene expression marker set (mammaglobin, cytokeratin 19) for detection of clinically actionable metastasis in breast SLNs using microarray methods. Furthermore, they developed a rapid RT-PCR assay (GeneSearch Breast Lymph Node [BLN] Assay; Veridex, Warren, NJ) using these two markers; it generates a result in less than 30 minutes. The authors assessed the utility of RT-PCR assay in SLNs of 254 breast cancer patients, reporting a sensitivity of 90% and specificity of 94%. Blumencranz and associates recently validated this RT-PCR assay in a prospective study at 11 clinical sites. The assay detected 98% of metastases larger than 2 mm and 88% of metastases larger than 0.2 mm. Micrometastases were less frequently detected (57%), and assay-positive results in nodes found to be negative by histology were rare (4%). The authors concluded that the use of the BLN assay overcomes conventional histologic sampling errors and can reduce the need for second surgeries to complete the axillary dissection on SLN-positive patients. Similar results have been reported by other authors who used this commercially available assay, and prospective trials are under way to further characterize its utility in daily practice. Other indices that portend an unfavorable prognosis for breast cancer relate to the presence of gross rather than microscopic disease in the lymph nodes and/or the presence of extranodal disease.
Evolution of Staging Systems
Early staging systems for breast cancer were based on the feasibility of operative intervention. Most tumors were classified as either operable or inoperable; however, this grouping did not offer any significant prognostic information. In 1905 the German physician Steinthal recommended three classifications for patients with breast cancer: (1) tumors not larger than “a plum” and clinically not associated with skin or axillary lymph node involvement, (2) large tumors adherent to the skin with palpable enlarged axillary lymph nodes, and (3) large tumors diffusely involving the breast with skin, deep muscle, and supraclavicular lymph node involvement. This classification was based on clinical factors that were perceived as important in predicting prognosis. Early on, it was clear that surgeons had identified some of the most ominous prognostic indicators for breast cancer. It is also interesting to note the inclusion of primary tumor size in this primitive staging scheme. An unpopular but insightful system was proposed by Lee and Stubenbord in 1928. Their system included an index of the rate of tumor growth. This method was the first to attempt assessment of the biology of individual tumors and their potential for progression.
In 1940 the four-stage Manchester classification was introduced ( Table 37.4 ). It permitted staging based solely on clinical criteria, including the extent of local involvement by the primary tumor, the presence and mobility of palpable enlarged axillary lymph nodes, and the presence of distant metastases. Neither pathologic information nor tumor size was included in this system. In 1943 Portmann described a staging system that incorporated clinical, pathologic, and roentgenographic characteristics of breast cancers and evaluated each lesion based on three categories: skin involvement, the location and mobility of the primary tumor, and the extent of local and distant metastases ( Table 37.5 ). Haagensen and Stout evaluated 568 patients with breast cancer who were treated with radical mastectomy. In 1943 they published the following criteria of inoperability, which were based on the clinical characteristics of patients who were clearly incurable by aggressive surgery alone:
- •
Extensive edema of the skin overlying the breast or edema of the arm
- •
Satellite nodules of the breast or parasternal tumor nodules
- •
Inflammatory carcinoma
- •
Supraclavicular or distant metastases
- •
Two or more of the five “grave signs” of locally advanced cancer:
- 1.
Breast skin edema
- 2.
Breast skin ulceration
- 3.
Tumor fixation to chest wall
- 4.
Axillary lymph node fixation to skin or deep tissues
- 5.
Enlarged axillary lymph nodes larger than 2.5 cm in diameter
- 1.
| Stage I | The tumor is confined to the breast. Involvement of the skin may be present, provided the area is small in relation to the size of the breast |
| Stage II | The tumor is confined to the breast and associated lymph nodes are present in the axilla |
| Stage III | The tumor extends beyond the breast as demonstrated by the following: |
| |
| |
| Stage IV | The tumor extends beyond the breast as shown by the following: |
| |
| |
| |
|
| Stage I | |
| – | Skin—not involved |
| + | Tumor—localized to breast, mobile |
| – | Metastases—none |
| Stage II | |
| – | Skin±not involved |
| + | Tumor—localized to breast, mobile |
| + | Metastases—few axillary lymph nodes involved in microscopic evaluation; no other metastases |
| Stage III | |
| – | Skin—edematous; brawny red induration and inflammation not obviously caused by infection; extensive ulceration; multiple secondary nodules |
| ++ | Tumor—diffusely infiltrating breast; fixation of tumor or breast to chest wall; edema of breast; secondary tumors |
| ++ | Metastases—many axillary lymph nodes involved or fixed; no clinical or roentgenologic evidence of distant metastases |
| Stage IV | |
| +/– | Skin—involved or not involved |
| +/++ | Tumor—localized or diffuse |
| +++ | Metastases—axillary and supraclavicular lymph nodes extensively involved; clinical or roentgenologic evidence of more distant metastases |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








