Chapter 3 Antiretroviral Therapy and Comprehensive HIV Care in Resource-Limited Settings
INTRODUCTION
In 2006, 39.5 million people worldwide were living with HIV, and over 4 million people were newly infected with the virus in 2006.1 Combating the AIDS pandemic requires a multi-sectoral, multi-faceted approach with a goal to ensure that countries everywhere come as close as possible to achieving universal access to HIV prevention, antiretroviral therapy (ART), and basic care. In resource-poor settings in Africa and Asia, where 90% of people with HIV/AIDS live, access to ART is limited. Over the past four years, the increasing availability of cheaper generic drug formulations and the launch of initiatives by international agencies, including the Global Fund to Fight AIDS, Tuberculosis, and Malaria and the United States President’s Emergency Plan for AIDS Relief (PEPFAR), have permitted expanded HIV-treatment programs to take root in many countries. As of December 2005, an estimated 1.3 million people were receiving ART in low- and middle-income countries. This represented only 20% of the estimated 6.5 million people in urgent need of antiretroviral therapy in those regions.2
There is compelling evidence that ART can be feasibly administered in resource-limited settings (RLSs) in both adults and children.3–5 Virologic and immunologic effectiveness, reduction in morbidity, and increased survival among patients in RLS are similar to those seen in Western countries.6–11 A review of multiple studies reported a substantial increase in CD4+ T-lymphocyte cell counts, and 73% of patients with an undetectable viral load by a median follow-up of 6 months).3 Many studies report high levels of treatment adherence that are comparable to or better than those of industrialized countries.12,13
Several factors could limit the effectiveness of ART in RLS, including: (1) patients often starting ART with advanced HIV infection, e.g., CD4+ T-lymphocyte cell counts <100 cells/μL)3; (2) a high prevalence of co-infections, including tuberculosis (TB) and bacterial diseases14–16; (3) Interruptions in treatment due to the high cost of antiretroviral (ARV) drugs or transportation to clinics17,18 (e.g., the percentage lost to follow-up in RLS ranges from 3.7% to 44%19); (4) maintaining an uninterrupted supply of drugs20,21; (5) monitoring the efficacy and safety of ARV treatment in countries with limited resources (which is problematic because of the lack of laboratory facilities that can conduct tests indicated for HIV care, such as CD4+ T-lymphocyte cell counts and viral loads, and even where such tests can be performed, most patients with HIV infection do not have access to or cannot afford them22); and (6) other challenges of ART, including organizational constraints, inadequate human resources, and limited healthcare infrastructure.
Comprehensive care (ART and non-ART) is important in preserving the health of HIV-infected persons and improving quality of life. Ideally, programs should implement care that includes prevention and treatment of opportunistic and other common illnesses, psychosocial support, and prevention of transmission of HIV. To optimize care and treatment for persons with HIV in Africa a two-pronged approach of ARV therapy and prophylaxis/treatment of opportunistic illness is important. Whereas many of the strategies regarding ART are similar regardless of place, the opportunistic infections (OIs) affecting adults and children with HIV vary depending on climate, environment, local infections, and economic conditions. For example, certain illnesses, such as malaria, are common in sub-Saharan Africa; at the same time, some of the infections discussed in current US-based guidelines,23 such as Mycobacterium avium complex, may be less common in some areas of Africa. Some interventions, such as provision of clean drinking water, are regularly provided by governmental services in the industrialized world, but are less available elsewhere. Because of these differences, it is useful to develop a locally applicable package of standard interventions that can be provided to patients with HIV. Cotrimoxazole (trimethoprim-sulfamethoxazole), which is inexpensive, widely available, and effective in reducing morbidity and mortality, should be the cornerstone of such a preventive care package.24
WHEN AND HOW TO INITIATE ART
When to Start ARV Treatment–WHO Eligibility Criteria
In April 2002, WHO published the first edition of the WHO ARV guidelines, a Public Health Approach, with simplified standardized tools for the delivery of ART. Updates of these guidelines have been published frequently, considering new scientific data and the experiences of increasing ART scale up in many countries.25 Modified versions of the WHO guidelines are generally implemented in RLS.3 The 2006 criteria for initiating ART are shown in Table 3-1.
BOX 3-1 Revised WHO Clinical Staging of HIV/AIDS for Adults and Adolescents
Clinical Stage III
Conditions where confirmatory diagnostic testing is necessary:
Table 3-1 WHO Clinical and Immunologic Criteria for Initiating ART
| Clinical Staging for Initiation of ART | ||
|---|---|---|
| Clinical Stage (see Revised WHO Clinical Staging, Box 3-1) | CD4+ T-lymphocyte Available | CD4+ T-lymphocyte Not Available |
| I | CD4+ T-lymphocyte guided | Do not treat |
| II | CD4+ T-lymphocyte guided | TLC guided |
| III | Consider CD4+ T-lymphocyte | Treat |
| IV | Treat | Treat |
| CD4+ T-lymphocyte Count Criteria for Initiation of ART | |
|---|---|
| CD4+ T-Lymphocyte Count (cells/μL) | Actions |
| <200 | Treat irrespective of clinical stage |
| 200–350 | Consider treatment, initiate before count drops below 200 cells/μL |
| >350 | Do not initiate treatment |
TLC, total lymphocyte count.
The majority of patients in the developing world who present for ART have CD4+ T-lymphocyte cell counts far below 200 cells/μL. Most of these patients receive a diagnosis of HIV infection only when they present to the hospital with a life-threatening OI.26 At the Infectious Diseases Institute clinic at Makerere University and Mulago Hospital in Kampala, Uganda, the median CD4+ T-lymphocyte cell count at the start of ART was 97.5 cells/μL for 918 patients who started therapy. There is urgent need to establish and scale up routine HIV testing in clinical and other settings to identify HIV-infected patients earlier in the course of HIV infection and to link them to HIV care programs.27
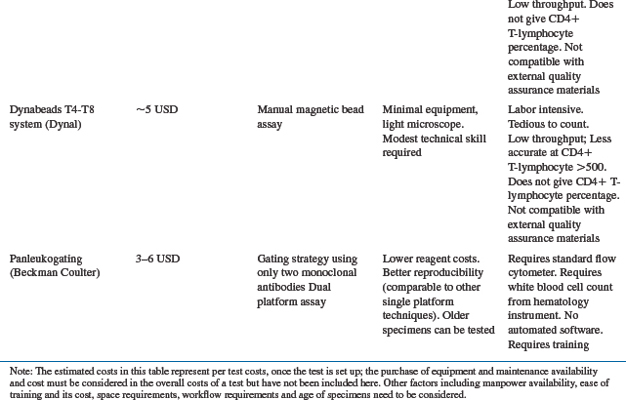
Evaluating the level of immune suppression is important for making decisions regarding initiation of ART and is a challenge in RLS. Flow cytometry serves as the gold standard technique to quantify the CD4+ T-lymphocyte cell population; however, other methods are also available. Table 3-2 shows the different methods of CD4+ T-lymphocyte testing (standard flow cytometry vs alternatives), and some of the advantages and disadvantages of each method. FACSCalibur equipment (Becton-Dickinson (BD) Biosciences, San Jose, CA, USA and EPICS XL, Beckman Coulter, Miami, FL, USA) is expensive but has the highest throughput and less cost per test, making it appropriate for central laboratories. The FACSCount System (BD) for measuring CD4+ T-lymphocyte cell counts is simpler to perform but requires more expensive reagents and therefore costs $5–10 US/test. One of the most crucial needs in the developing world is universal access to affordable and locally usable CD4+ T-lymphocyte testing technology. As access to ART increases worldwide, alternative laboratory monitoring methods are being considered for use in RLS. For example, the Guava Easy CD4+ T-lymphocyte assay (Guava Technologies, Hayward, CA, USA) utilizes microcapillary cytometry for CD4+ T-lymphocyte cell enumeration. Due to small reagent amounts and no requirement for sheath fluid, costs can be minimized–about $1.25 per test. However, the relatively low throughput of the Guava means that this technology has to be applied in smaller health centers with lower demand for CD4+ T-lymphocyte testing. Josefowicz et al conducted a five-site evaluation in the US and Canada and demonstrated that the Easy CD4+ T-lymphocyte assay is precise and accurate when compared to flow cytometry.28 Our recent study showed that the CD4+ T-lymphocyte cell count determined by the GuavaTech Easy CD4+ T-lymphocyte was comparable to that determined by flow cytometry, with a sensitivity and specificity for CD4+ T-lymphocyte below 200 cells/μL of 90% and 98%, respectively.29 Low-cost manual methods for CD4+ T-lymphocyte cell enumeration such as the Dynabeads technique30–32 now exist. Studies evaluating the performance of the Dynabeads methods for the determination of CD4+ T-lymphocyte cells show high overall correlation coefficients between Dynabeads and FACSCount.31,33 Since the Dynabeads method is simpler and cheaper than flow cytometry, it provides an alternative option for the enumeration of CD4+ T-lymphocyte cells in laboratories with limited facilities. However, the manual methods (Dynabeads, Cytospheres) are much more labor intensive, and their application in ART scale-up in RLS is less clear. Considerations in making decisions regarding choice of CD4+ T-lymphocyte testing method besides instrument and reagent costs include training requirements, throughput, equipment maintenance, supply chain, compatibility with proficiency testing materials, and technician time.
Studies from Africa show simple markers such as HIV-related symptoms (e.g., WHO clinical stage 4); anemia (e.g., hemoglobin less than 8 g/dL) and total lymphocyte count (TLC) (e.g., less than 1200 cells/μL) are relatively specific for a CD4+ T-lymphocyte cell count <200 cells/μL and can be used to determine initiation of ART; however, they are not sensitive predictors of CD4+ T-lymphocyte cell counts below 200 cells/μL.34,35 The TLC cutoff point has major impact on the sensitivity and specificity of this marker in predicting CD4+ T-lymphocyte cell counts.36 A higher cutoff increases sensitivity, reducing the likelihood of failing to identify those patients who might benefit from ART. With a lower cutoff (e.g., 1000–1200 cells/μL), sensitivity is lost, but specificity increases.35,36 In RLS, a lower TLC cutoff point with maximal specificity may be a more practical and cost-effective public health approach for determining eligibility for initiation of ART in asymptomatic or mildly symptomatic HIV-infected patients where CD4+ T-lymphocyte testing is unavailable.36 In RLS (as well as in industrialized countries), the clinical advantages of starting treatment at CD4+ T-lymphocytecell counts above 200 cells/μL are unclear.37
Viral load testing is very costly and is not of much benefit for initiating ART, even in industrialized countries. It should not be part of screening algorithms for initiating ART in RLS.38
How to Start ART
Baseline Clinical Assessment
Before any patient is started on ART, he/she should undergo baseline clinical assessment including a medical history, physical examination, laboratory investigations, staging of disease (WHO clinical criteria) and counseling and assessment of patients’ readiness to start therapy. The treatment of coexisting infection takes priority over starting ART. ART should not be started in patients with serious co-morbidities or those who are terminally ill. However, in most HIV treatment centers in RLS, the facilities to diagnose OIs are limited. Particularly, in countries with a high burden of TB (which includes most middle- and low-income countries), ART programs are challenged with diagnosing TB. In our experience, ART initiation commonly causes the unmasking of occult TB.39 The rolling out of ART therefore requires that TB diagnostic capacity be strengthened. TB screening protocols should be part of any ART program in endemic areas.
The cost-effectiveness of performing baseline serum chemistry tests (liver and renal function tests) at baseline in RLSs is not clear, but studies to answer this question are ongoing.40 In Uganda, 11% of patients beginning ART had a positive serum cryptococcal antigen (CRAG) test at baseline.41 About two-thirds of patients with a positive CRAG test who did not have a prior history of cryptococcal infection developed cryptococcal meningitis while on ART. However, there are no specific data on the impact of an intervention of routine screening and treatment of CRAG-positive asymptomatic persons on morbidity or mortality. Table 3-3 summarizes a baseline clinical evaluation checklist for patients starting ART.
Table 3-3 Baseline Clinical Evaluation Checklist for Patients Starting ART
Treatment Education and Assessment of Readiness to Start
CHOICE OF ART REGIMENS IN ADULTS AND ADOLESCENTS
Table 3-4 shows the first- and second-line ARV regimens in adults and adolescents recommended by WHO in 2006.42 Most countries have their own guidelines that are generally derived from the WHO guidelines.
Table 3-4 First- and Second-Line ARV Drug Regimens in Adults and Adolescents (2006) as Recommended by WHO42
| Second-Line Regimens | ||
|---|---|---|
| First-Line Regimens | RTI Componenta | PI Componentb |
| (ZDV or d4T) + (3TC or FTC) + (EFV or NVP) | ABC + ddI or | ATV/r or |
| ABC + TDF or | LPV/r or | |
| TDF + 3TC ± ZDV | SQV/r | |
| TDF + (3TC or FTC) + (EFV or NVP) | ABC + ddI or | ATV/r or |
| ddI + 3TC ± ZDV | LPV/r or | |
| SQV/r | ||
| ABC +(3TC or FTC) + (EFV or NVP) | ddI + 3TC ± ZDV | ATV/r or |
| LPV/r or | ||
| or | SQV/r | |
| TDF + 3TC ± ZDV | ||
| (ZDV or d4T) + (3TC or FTC) + (ABC or TDF) | EFV or NVP ± ddI | |
ZDV, zidovudine; d4T, stavudine; 3TC, lamivudine; FTC, emtricitabine; EFV, efavirenz; NVP, nevirapine; ABC, abacavir; ddI, didanosine; TDF, tenofovir; ATV/r, ritonavir-boosted atazanavir; LPV/r, ritonavir-boosted lopinavir; SQV/r, ritonavir-boosted saquinavir.
a 3TC can be considered to be maintained in second-line regimens to reduce viral fitness.
b Nelfinavir (NFV) or ATV in places without cold chain. TDF cannot be used with unboosted ATV.
Choices for First Line
Rationale for Choice of Initial ART Regimens
Currently the initial treatment regimens that are widely used in RLS are: non-nucleoside reverse transcriptase inhibitor (NNRTI): EFV or NVP plus a nucleoside reverse transcriptase inhibitor (NRTI) backbone: stavudine (d4T) plus lamivudine (3TC) or ZDV plus 3TC. These first-line regimens prolong life, have a low pill burden, and have the lowest cost at the present time. The current treatment regimens permit rapid scale-up. However, they are also associated with drug toxicities that may be irreversible or lethal.43 Patients at highest risk of these toxicities are those with advanced disease who are often given the highest priority in ART programs in RLS. Currently the data on the incidence and severity of ARV drug toxicities in African patients are still limited.
Nucleoside Reverse Transcriptase Inhibitors
Concerns are increasingly being voiced about the widespread use of stavudine (d4T), given its propensity to cause a progressive disabling peripheral neuropathy especially among patients with advanced HIV and preexisting peripheral neuropathy26 and those taking concomitant anti-TB drugs.44 An ongoing program in rural Uganda is following a cohort of 1029 adult patients on a stavudine-containing regimen. Thirty-six per cent of the patients developed neuropathy (9% severe grade) after 11,268 patient months of observation.45 Stavudine is also associated with stigmatizing facial lipoatrophy,46 limb fat loss, and lactic acidosis (rare but potentially fatal). Since stavudine is widely used in first-line regimens, patients on stavudine should be monitored closely for neuropathy and lipoatrophy and should be switched to ZDV in a timely fashion to minimize effects of stavudine cumulative toxicity.
Zidovudine also is not without problems; ZDV is associated with anemia, which may lead to blood transfusions and rarely to death. In the DART trial, 21% of patients on ZDV developed a new episode of anemia following initiation of therapy, and 6.6% developed new grade-4 anemia by week 48.47
Non-Nucleoside Reverse Transcriptase Inhibitors
Using the fixed-dose combination (currently the least expensive regimen) of d4T, 3TC, and NVP poses a complication with regard to starting and stopping treatment. The recommended NVP dosing regimen starts with a lower lead-in dose of 200 mg q.d. for 2 weeks, followed by 200 mg b.i.d. thereafter. This is based on analyses suggesting that this regimen is less frequently associated with rash. Starting a fixed-dose regimen of combination NNRTI–NRTI treatment without the ‘lead in’ dose of NVP may be associated with increased toxicity. In addition, NVP has a very long pharmacokinetic tail after discontinuation, and a low genetic barrier to resistance. Therefore, stopping use of the fixed-dose combination without continuing the NRTIs for an additional 5 days may lead to NNRTI resistance.49
Concern about the use of NNRTIs as first-line ARV treatment is growing because of the increased use of NVP monotherapy to prevent mother-to-child transmission of HIV infection.50 One dose of NVP during delivery has been shown to cause significant resistance mutations.51,52 Johnson et al53 showed that resistance mutations emerge in at least 65% of the women after single dose NVP. In a study in Thailand, women who received intrapartum NVP were less likely to have virologic suppression after 6 months of postpartum treatment with an NVP-containing regimen than those who had not received NVP.50 The HIV-1 RNA level was less than 50 copies/mL in 49% of the women who had received intrapartum NVP, compared with 68% of the women who had not received intrapartum NVP (P = 0.03). More recent data do not support an adverse impact of NVP monotherapy for PMTCT on subsequent response to NVP-based ART if ART is initiated 6 months or more after receipt of a single, peripartum dose of nevirapine.54 Further research to determine clinical implications is needed.
Regimens that contain EFV should not be used by women at risk of pregnancy because of the teratogenic potential for the fetus.55 The potential teratogenicity of EFV means that effective contraception is strongly recommended for women taking this drug. Despite the increasing attention to ART in RLS, support for reproductive choice in HIV-infected individuals has lagged behind.56 More research is needed to understand reproductive decisions in HIV-infected women and the factors that influence them. Because of drug interactions with rifampin, the use of NVP is generally avoided in patients who require both TB and ART.57
PI-Based Regimens
PI-based regimens are an accepted standard of care for initial regimens. However, their high cost relative to NNRTI-based regimens makes their use problematic in RLS seeking to achieve rapid scale-up of therapy. Disadvantages are higher pill counts, food and water requirements in some cases, significant interactions with other drugs that preclude or complicate their use with TB treatment regimens including rifampin, metabolic abnormalities, and the need for a functioning cold chain for ritonavir (RTV)-boosted regimens. In RLS, PIs are generally reserved for second-line therapy.24
Triple NRTIs
The triple-nucleoside combination of ABC, ZDV, and 3TC was associated with an inferior virological outcome, compared with a regimen containing EFV and two or three nucleosides.58 However, emerging data suggest that select triple NRTI regimens are simple to administer, have low pill burden, are associated with acceptable HIV RNA reductions and CD4+ T-lymphocyte cell increases, are relatively safe, have fewer drug–drug interactions and are less likely than NNRTI-containing regimens to cause ARV drug resistance when treatment is interrupted. These regimens are being studied59 and may be good choices for patients with TB or in whom a preferred or alternative NNRTI- or PI-based regimen may be less desirable due to concerns about toxicity, drug interactions, or regimen complexity. Examples of triple NRTI regimens include: ZDV + 3TC + TDF; ZDV + 3TC + ABC.
MONITORING AND SWITCHING ART
Once patients have been started on ART, they must be monitored for efficacy and toxicity. In developed countries, the efficacy of an ARV treatment regimen is monitored by viral load testing, and decisions to switch therapy are based on increasing viral load, decreasing CD4+ T-lymphocyte cell counts and resistance testing.60 Western style monitoring is not affordable and is severely limited by laboratory infrastructure in RLS. Unlike the prices for ARV drugs, the prices for laboratory reagents have not decreased significantly in recent years, although there are efforts to find new and less expensive ways to monitor efficacy of treatment.61,62 The lack of laboratory monitoring should not prevent initiating or continuing ART. In fact, the minimum laboratory testing (in addition to clinical monitoring) that is adequate for monitoring for drug toxicity in RLS is not known. Several studies looking at the effect of different levels of monitoring on patient health outcomes are underway.40,59 In the meantime, WHO recommends clinical monitoring, with symptom- and ART drug regimen-directed laboratory assessment as needed for monitoring ART.
Clinical Monitoring
Using clinical symptoms to assess the possibility of treatment failure is a challenge and may be inaccurate, especially during the first 3–6 months of ART. Patients may develop symptoms that are not caused by treatment failure but instead represent the side effects of ARV therapy, an immune reactivation inflammatory syndrome,63 OIs that continue to appear because the patient is still immunocompromised, or an infection/reinfection by a common endemic pathogen such as TB or malaria. If a patient has been adherent with a good ART regimen and has not had previous ARV exposure, very few treatment failures will occur during the first 6 months.64 After 6 months to 1 year of ART, clinical manifestations will be more useful for predicting treatment success/failure. This will be particularly so in patients who were symptomatic (WHO clinical stage III and IV) at the start of ART, a group that currently represents the majority of those who start ART in countries with limited resources. Development of a new OI or loss of weight may signal treatment failure. Following the effect of ART on relatively mild HIV-related symptoms and signs such as prurigo may be useful markers of treatment response but remain unproven. Prurigo occurs in at least 10% of African patients with advanced HIV disease.65 Once prurigo appears, in the absence of ARV treatment the itching and the papular eruption generally persist and symptomatic treatment is ineffective. In a Ugandan study, pruritic papular eruption significantly improved and in some cases disappeared among patients with advanced HIV infection started on ART (Colebunders Robert, Byakwaga Helen, personal observation).
Immunological Monitoring
CD4+ T-lymphocyte cell counts are useful in detecting asymptomatic patients or patients with minor symptoms (WHO stage-II disease) who require ART. A CD4+ T-lymphocyte cell count may also help in deciding when to start or stop prophylaxis for OIs but is of less value as an indirect tool to monitor antiviral efficacy. Indeed, in most instances, once a patient has failed immunologically (or clinically), viral resistance has already evolved. Moreover, the change in CD4+ T-lymphocyte cell count may vary from one patient to another regardless of the virological efficacy of a regimen. A slight increase in CD4+ T-lymphocyte cell count (<50 cells/μL after 1 year of ART) does not necessarily imply treatment failure.66,67 Conversely, a patient on a virologically failing regimen whose virus is resistant to only one or two drugs of the regimen may have a continued increase in CD4+ T-lymphocyte cell count.68,69 The criteria proposed by the WHO for immunological treatment failure are (1) a CD4+ T-lymphocyte cell count below 100 cells/mm3 after six months of therapy; (2) a return to, or a decrease to below the pre-therapy CD4+ T-lymphocyte cell count after six months of therapy; or (3) a 50% decline from the on-treatment peak CD4+ T-lymphocyte cell value (if known).42 Ideally, CD4+ T-lymphocyte cell count testing should be done when the patient does not have an active OI since intercurrent infections may cause CD4+ T-lymphocyte cell count levels to decline.70 A problem in resource poor settings is that many OIs are difficult to diagnose. Therefore, in a patient who is not doing well clinically, with a decreasing CD4+ T-lymphocyte cell count, it is often unclear whether this is because of an intercurrent illness, HIV disease progression, or both.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree