Fig. 17.1
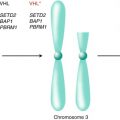
RCC biology which leads to VEGF upregulation. Therapeutics are listed in the red boxes that target major elements of this pathway
17.3 Inhibition of VEGF in Renal Carcinoma
The above data provide evidence for VHL gene inactivation in the majority of clear cell RCC tumors, which leads to overexpression of VEGF and other factors as a driving force in renal tumor angiogenesis. In fact, RCC develops highly vascular features in both the primary and metastatic sites of disease. Thus, with the development of effective agents targeting the angiogenesis signaling pathway, inhibition of VEGF has been aggressively pursued as a therapeutic target in RCC.
17.3.1 Sorafenib
Sorafenib (Nexavar®, Onyx Pharmaceuticals, Inc., and Bayer Pharmaceuticals Corp.) is an inhibitor of VEGF receptor 2, FLT3, c-Kit, platelet-derived growth factor receptor (PDGF-R), fibroblast growth factor receptor-1 (FGF-R-1), CRAF, and both mutant and wild-type BRAF [18]. It received FDA approval on December 20, 2005. This approval was granted based on the results of a phase III study in 905 patients with advanced renal cell carcinoma who had received one prior systemic treatment with endpoints of overall survival (OS), progression-free survival (PFS; primary endpoint), and response rate. Patients with ECOG performance status (PS) of 0 or 1, and favorable or intermediate Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk category were eligible for enrollment. Sorafenib improved the median progression-free survival (PFS) to 5.5 months vs. 2.8 months in the placebo group (HR = 0.44; 95 % CI, 0.35–0.55; p < 0.001). The observed benefit in progression-free survival (PFS) was independent of age, MSKCC score, previous use of cytokine therapy, presence of lung or liver metastases, as well as the time since diagnosis (<1.5 or ≥1.5 years). The median overall survival in the sorafenib group in this trial was 19.3 months vs. 15.9 months in the placebo group (HR = 0.77; 95 % CI, 0.63–0.95; p = 0.02); however, this result did not reach the pre-specified O’Brien–Fleming boundaries for statistical significance [19]. However, after censoring the placebo patients who crossed over to the [sorafenib] arm, there was a suggestion of improved OS with sorafenib (17.8 versus 14.3 months; p = 0.029).
Among the 451 patients assigned to sorafenib (of the total of 903 patients in the trial), 18 patients (4 %) discontinued therapy for adverse events. The most common adverse events were diarrhea (43 %), rash (40 %), fatigue (37 %), hand–foot syndrome (30 %), nausea (23 %), alopecia (27 %), pruritus (19 %), and hypertension (17 %). Anemia was reported in 8 % of the patients receiving sorafenib [19].
A clinical trial of sorafenib 400 mg twice daily vs. INF-α in first-line treatment of mRCC was conducted to further explore the activity of sorafenib in the frontline setting. Sorafenib did not show any PFS benefit (5.7 months in sorafenib vs. 5.6 months in INF-α; HR = 1.14; 95 % CI, 0.79–1.64; p = 0.504). However, patients on sorafenib had better quality of life indices. Additionally, on dose escalation to 600 mg twice daily after progression of disease (PD) on the lower dose (400 mg twice daily), there was an additional PFS of 3.6 months. Patients who had PD on INF-α crossed over to sorafenib 400 mg twice daily and had an additional PFS of 5.3 months [20]. These data have tempered the enthusiasm for sorafenib in the frontline setting, although it is still a viable option owing to the overall good tolerability.
With other drugs having shown superior efficacy in first-line and second-line setting, sorafenib’s use has been relegated to individuals who have had disease progression after being on newer VEGF-R-targeted therapy. More recently, sorafenib was compared to temsirolimus, an MTOR inhibitor, in a second-line setting for patients who had disease progression on sunitinib. Sorafenib demonstrated superior OS (stratified HR, 1.31; 95 % CI, 1.05–1.63; two-sided p = 0.01) and median OS in sorafenib and temsirolimus arms was 16.6 months and 12.3 months. The safety profiles of these drugs were similar to those seen in previous studies [21]. While the precise reason for the OS advantage to sorafenib is unclear, these data have largely been interpreted to support the concept of sequential VEGF-targeting therapy in metastatic RCC.
17.3.2 Sunitinib
Sunitinib (Sutent®, Pfizer, Inc.) is a potent inhibitor of VEGF-R types 1–3, FLT3, c-Kit, PDGF-R-α, and PDGF-R-β [22]. It received FDA approval on January 26, 2006. Two initial phase II trials of sunitinib (50 mg/day for 4 weeks followed by 2 weeks rest) in a total of 169 metastatic RCC patients who had failed prior cytokine-based therapy demonstrated an investigator-assessed objective response rate of 45 %, a median duration of response of 11.9 months, and a median PFS of 8.4 months [23, 24]. This was later converted to regular approval based on an improvement in progression-free survival (PFS) in a randomized phase III first-line therapy setting [25]. Previously untreated mRCC patients (n = 750) with clear cell histology were randomized 1:1 to receive sunitinib 50 mg once daily, in 6-week cycles consisting of 4 weeks of treatment followed by 2 weeks without treatment or IFN-α as a subcutaneous injection three times per week on nonconsecutive days at 3 MU per dose during the first week, 6 MU per dose the second week, and 9 MU per dose thereafter. The primary endpoint was PFS (from historical control of 4.7–6.2 months). Secondary endpoints included objective response rate, overall survival, and safety. Health-related quality of life was also assessed with the use of the Functional Assessment of Cancer Therapy–General (FACT-G) and FACT–Kidney Symptom Index (FKSI) questionnaires. Patients were stratified according to baseline levels of LDH, ECOG performance status, and the presence or absence of nephrectomy. The objective response rate by investigator review was 47 % in the sunitinib group versus 12 % in the IFN-α group; p < 0.001. Similarly, the median PFS by third-party independent review was 11 months versus 5 months in favor of sunitinib-treated patients corresponding to an HR of 0.42 (95 % CI, 0.32–0.54; p < 0.001). Sunitinib-treated patients had a greater median OS when compared with the IFN-α group (26.4 months; 95 % CI, 23.0–32.9 months, versus 21.8 months; 95 % CI, 17.9–26.9 months, respectively; HR, 0.821; 95 % CI, 0.673–1.001; p = 0.051) based on the primary analysis of the unstratified log-rank test (p = 0.013 using the unstratified Wilcoxon test). By stratified log-rank test, the HR was 0.818 (95 % CI, 0.669–0.999; p = 0.049). More than 50 % of patients in both arms of this trial went to receive subsequent treatment with a VEGF-targeted agent including sunitinib, perhaps accounting for the lack of statistical significance observed in the pre-specified OS analysis. The results of this trial have positioned sunitinib as a standard frontline therapy for mRCC patients. The main limitation of the approved regimen of a 6-week cycle of 50 mg/day for 4 weeks followed by 2 weeks off therapy was toxicity. Seventy patients (19 %) in the sunitinib treatment arm (N = 375) discontinued treatment for adverse events. Diarrhea, fatigue, and nausea were seen in more than 50 % and hypertension and hand–foot syndrome in approximately 30 % of patients on sunitinib. Laboratory abnormalities included anemia (79 %), neutropenia (77 %), and thrombocytopenia (68 %) [26].
A recent randomized phase II trial examined the standard dosing of sunitinib (Arm A) vs. continuous dosing at 37.5 mg daily (Arm B) [27] in the first-line management of mRCC. The primary endpoint was time to tumor progression (TTP) and the secondary endpoints included objective response rate (ORR), overall survival (OS), and adverse events. Two hundred and ninety-two patients were randomized equally to both arms. Median TTP was 9.9 vs. 7.1 months in Arms A and B, respectively (HR = 0.773; 95 % CI, 0.57–1.04; p = 0.090). ORR and OS were not statistically significantly different, although numerically favored the 50 mg 4/2 regimen. The most common adverse events were fatigue 65 % vs.71 % in both groups, nausea 63 % vs. 54 %, and diarrhea 59 % vs. 69 % [28]. These data support that 50 mg 4/2 is the preferred dose and schedule and that lower doses do not improve tolerability and may compromise clinical outcome. Alternative schedules of sunitinib such as 2 weeks on followed by 1 week off have been shown to decrease toxicity and better balance the benefits and side effects of this agent with planned prospective trials of alternative schedules [29].
To study the role of sunitinib in the second-line setting for mRCC patients who had failed prior bevacizumab-based therapy, a small (n = 61) phase II trial was conducted [30]. Tumor burden reduction was observed in 85 % of patients including 14 patients (23 %) who achieved a RECIST-defined PR. The median PFS was 30.4 weeks (95 % CI, 18.3–36.7 weeks) and median OS was 47.1 weeks (95 % CI, 36.9–79.4 weeks). In this study, prior response to bevacizumab did not predict for subsequent response or lack thereof to second-line sunitinib treatment. These data support the empiric current practice of sequential VEGF-targeted monotherapies in metastatic RCC patients.
17.3.3 Pazopanib
Pazopanib (Votrient™, GlaxoSmithKline) is an oral angiogenesis inhibitor with multiple targets including vascular endothelial growth factor receptor (VEGF-R), platelet-derived growth factor receptor (PDGF-R), and c-Kit. It received FDA approval on October 19, 2009, after a phase I clinical trial established the MTD and DLT of pazopanib in refractory solid tumors [31]. A multicenter phase II trial examined the efficacy and safety of pazopanib (800 mg orally daily) in 225 mRCC patients [32]. This study was originally designed as a randomized discontinuation trial. However, after planned interim analysis conducted after the first 60 patients completed 12 weeks of treatment demonstrated a response rate of 38 %. Based on this activity and on recommendation by the independent DSMB, randomization was halted, and all continuing patients in the study were treated on an open-label basis. The ORR observed was 35 % (95 % CI, 28–41 %) by independent review. This was similar regardless of previous treatment or not (37 % versus 34 %, respectively). The estimated median PFS for the entire cohort was 45 weeks (95 % CI, 36–59 weeks). Although the toxicity profile was similar to that seen with other small VEGF-R inhibitors, grade 3 AST and ALT elevation were noted in 6 % and 4 %, respectively, and have emerged as a somewhat unique side effect to this agent.
FDA approval was granted based on a randomized placebo-controlled phase III trial in 435 patients previously untreated or treated with cytokine therapy; most patients were good or intermediate risk group. This clinical trial found that pazopanib compared to placebo significantly prolonged PFS in the overall study population (median PFS 9.2 vs. 4.2 months; HR = 0.46; 95 % CI, 0.34–0.62; p < 0.0001), in the treatment-naïve subpopulation (median PFS 11.1 vs. 2.8 months; HR = 0.40; 95 % CI, 0.27–0.60; p < 0.0001), and in the cytokine-pretreated subpopulation (median PFS 7.4 vs. 4.2 months; HR = 0.54; 95 % CI, 0.35–0.84; p < 0.001). The objective response rates in this clinical trial were 30 % in the pazopanib group vs. 3 % in the placebo group with a median duration of responses of 59 weeks [33]. Among the 290 patients assigned to pazopanib (of the total of 435 patients in the trial), 41 patients (14 %) discontinued therapy for adverse events. The most common adverse events were diarrhea (52 %), hypertension (40 %), hair color changes (38 %), nausea (26 %), and fatigue (19 %). Abnormal ALT and AST (53 %), hyperglycemia (41 %), neutropenia (34 %), and thrombocytopenia (32 %) were among the more common laboratory abnormalities reported with use of pazopanib [33].
Pazopanib and sunitinib were compared head to head in a first-line setting in the COMPARZ trial, a non-inferiority trial for patients with metastatic clear cell renal cell carcinoma. One thousand one hundred patients were randomized to receive either pazopanib 800 mg daily or sunitinib 50 mg daily (4-week-on, 2-week-off schedule). Pazopanib was shown to be non-inferior with respect to PFS (HR, 1.05; 95 % CI, 0.90–1.22). Overall survival was found to be similar (HR, 0.91; 95 % CI, 0.76–1.08). Patients treated with sunitinib had higher incidence of fatigue (63 % vs. 55 %), hand–foot syndrome (50 % vs. 29 %), and thrombocytopenia (78 % vs. 41 %). Patients on pazopanib were more likely to have abnormalities in liver function tests (ALT elevations 60 % vs. 43 %). In 11 out of 14 health-related quality of life domains, treatment favored pazopanib (p < 0.05) [34].
Pazopanib and sunitinib were also compared in a randomized, controlled, double-blind crossover trial (PISCES) looking at patient preference. Pazopanib was preferred to sunitinib by patients (70 % vs. 22 %) with less fatigue and overall better quality of life being the main factors [35]. These data have led to some increase in the frontline use of pazopanib although sunitinib is still more commonly used. The data show that both agents are effective in this setting with some differences in tolerability that may allow for individualization of therapy.
17.3.4 Bevacizumab
Bevacizumab (Avastin®, Genentech, Inc.) is a monoclonal antibody that binds to and neutralizes circulating VEGF. It received FDA approval on July 31, 2009, in combination with interferon alpha (IFN-α) for the treatment of patients with metastatic renal cell carcinoma. The approval was based on the results from two multicenter phase III clinical trials of previously untreated patients with metastatic renal cell carcinoma. The AVOREN study was an international phase III trial that randomized 649 untreated mRCC patients to receive treatment either with IFN-α (Roferon; Roche, Basel, Switzerland) plus placebo or interferon plus bevacizumab [36]. Patients had predominant (>50 %) clear cell histology and had undergone a previous nephrectomy. Bevacizumab 10 mg/kg or placebo was administered intravenously every 2 weeks with no dose reductions permitted. IFN-α 9 MIU was administered three times per week as a subcutaneous injection. The study was designed to detect an OS improvement from 13 to 17 months with PFS, ORR, and safety as secondary endpoints. Due to the change in standard of care and the availability of other active VEGF inhibitors which precluded reaching the anticipated OS endpoint, the study was amended and unblinded at the time of final PFS analysis. The median PFS observed was 10.2 months in the bevacizumab plus IFN-α group, compared with 5.4 months in the control group (HR, 0.63; 95 % CI, 0.52–0.75; p = 0.0001); a significant ORR difference was also observed in favor of the bevacizumab-treated patients (31 % vs. 13 %; p < 0.0001). The final median OS was 23.3 months in the bevacizumab arm compared to 21.3 for the IFN-α plus placebo-treated arm (HR, 0.86; 95 % CI, 0.72–1.04; stratified log-rank test p = 0.1291).
A second multicenter phase III trial, which was conducted in the United States and Canada through the Cancer and Leukemia Group B (CALGB 90206) [37, 38], was nearly identical in design with the exception that it lacked a placebo infusion and did not require prior nephrectomy. This trial enrolled 732 untreated mRCC patients (369 to bevacizumab plus IFN-α and 363 to IFN-α alone). The primary endpoint of the study was to detect a 30 % improvement in OS in patients randomly assigned to bevacizumab plus IFN-α compared to IFN-α monotherapy. The median PFS of the study was 8.5 months in patients who received bevacizumab plus interferon versus 5.2 months for patients who received interferon monotherapy (p < 0.0001). The hazard ratio for progression in patients who received bevacizumab plus interferon after adjusting for stratification factors was 0.71 (p < 0.0001). Moreover, among patients with measurable disease, the ORR was higher in patients who received bevacizumab plus interferon (25.5 %) than for patients who received IFN-α monotherapy (13.1 %; p < 0.0001). The median OS in this study was 18.3 months for bevacizumab-treated patients compared to 17.4 months for those receiving IFN-α alone (p = 0.069). The contribution of interferon to the antitumor effect of this regimen currently is unclear as neither study contained a bevacizumab monotherapy arm, precluding evaluation of the risk/benefit of the addition of cytokines. Similarly, the appropriate dose of IFN-α when given in combination with bevacizumab remains unknown, notwithstanding the fact that a significant percentage of patients receiving the bevacizumab-containing regimen in both phase 3 trials required dose modifications of IFN-α. A recent exploratory analysis of the AVOREN study would suggest that the improvement of PFS observed with the addition of the VEGF antibody to IFN-α appears to be maintained in spite of the need for IFN-α dose reductions (10.2 months with full dose vs. 12.4 months in patients who required a reduced dose of IFN-α) [36]. Given the lack of dose response for interferon, it is possible that lower interferon doses in this combination can reduce toxic effects and preserve efficacy. Such a hypothesis requires prospective testing.
Among the 325 patients assigned to bevacizumab (of the total of 649 patients in the trial), 86 patients (26 %) discontinued therapy for adverse events. The most common adverse events were pyrexia (45 %), anorexia (36 %), fatigue (33 %), bleeding (33 %), asthenia (32 %), hypertension (26 %), flu-like illness (24 %), and diarrhea (20 %). Proteinuria (18 %) and neutropenia (7 %) were among the more common laboratory abnormalities reported with use of bevacizumab. The use of bevacizumab as frontline therapy has been limited by the need for IV infusion and the phase III data which supports the concomitant use of IFN-α.
17.3.5 Axitinib
Axitinib (Inlyta® Pfizer, Inc) is an oral selective inhibitor of vascular endothelial growth factor receptors (VEGF-R) 1, 2, and 3. Data from a multicenter, open-label, phase II study of patients with sorafenib-refractory mRCC who received a starting dose of axitinib 5 mg orally twice daily with a primary endpoint of objective response rate (ORR) provided evidence of activity of axitinib in this disease. Out of 52 patients which enrolled, 2 complete and 21 partial responses were seen with an ORR of 44.2 % (95 % CI, 30.5–58.7). Median response duration was 23.0 months (20.9, not estimable; range 4.2–29.8). Median time to progression was 15.7 months (8.4–23.4, range 0.03–31.5) [39].
In another phase II trial, 62 patients were recruited and the ORR was 22.6 %, and the median duration of response was 17.5 months. The median PFS was 7.4 months (95 % CI, 6.7–11.0 months), while the median OS was 13.6 months (95 % CI, 8.4–18.8 months). Grade 3–4 adverse events included hand–foot syndrome (16.1 %), fatigue (16.1 %), hypertension (16.1 %), dyspnea (14.5 %), diarrhea (14.5 %), dehydration (8.1 %), and hypotension (6.5 %) [40].
Axitinib gained FDA approval in 2012 based on a study where it was compared to sorafenib in a phase III trial with patients who had mRCC refractory to one prior first-line regimen (AXIS trial). Seven hundred twenty-three patients were randomly assigned to receive either axitinib (5 mg bid n = 361) or sorafenib (400 mg bid n = 362). Axitinib was associated with a more favorable PFS (6.7 months vs. 4.7 months; HR, 0.665; 95 % CI, 0.544–0.812; one-sided p < 0.0001). Common side effects were diarrhea, hypertension, dysphonia, and nausea seen in the axitinib arm and HFS, diarrhea, and alopecia in the sorafenib arm [41].
A separate phase III trial in treatment-naïve or cytokine-refractory metastatic RCC patients compared axitinib to sorafenib in metastatic RCC, and it did not show a significant improvement in PFS (10.1 months vs. 6.5 months; stratified HR, 0.77; 95 % CI, 0.56–1.05). Any-grade adverse events that were more common (≥10 % difference) with axitinib than with sorafenib were diarrhea (50 % vs. 40 %), hypertension (49 % vs. 29 %), weight decrease (37 % vs. 24 %), decreased appetite (29 % vs. 19 %), dysphonia (23 % vs. 10 %), hypothyroidism (21 % vs. 7 %), and upper abdominal pain (16 % vs. 6 %) [42]. Individualized dose titrations of axitinib (5–7 mg and then to 10 mg) in select patients were shown to result in a higher proportion of objective responses (54 % vs. 34 %) as compared to a placebo titration in a recent randomized double-blind phase II trial [43]. The data from these trials establish axitinib as second-line treatment in metastatic RCC with individualized dose titration resulting in a higher rate of objective responses. The optimal method for selecting patients for titration and the scheme by which to titrate require further study.
17.3.6 Cediranib
Cediranib (AstraZeneca) is an oral pan-inhibitor of vascular endothelial growth factor receptors (VEGF-R). In a multicenter, open-label phase II clinical trial, 44 previously untreated patients with mRCC were treated with cediranib 45 mg orally daily, titrated according to tolerance. The primary endpoint of the trial was RECIST-defined objective response (OR). In the 39 patients that were evaluable for response, partial response was observed in 15 (38 %) and stable disease in 18 patients (47 %). Overall tumor control rate was 84 % (95 % CI, 67–95 %). The median PFS was 8.9 months (95%CI, 5.1–12.9). Treatment-related grade 3 or greater adverse events included hypertension (36 %), fatigue (30 %), HFS (16 %), diarrhea (11 %), and anorexia (9 %). Authors concluded that cediranib has substantial antitumor activity in a first-line setting. However, a dose of 45 mg resulted in a higher incidence of grade 3 toxicities or higher and a number of patients had to have dosage adjustments [44].
A double-blind, placebo-controlled study of cediranib in patients with metastatic or recurrent RCC randomized patients 3:1 to cediranib 45 mg/day or placebo. The primary objective was to determine the efficacy judged by changes in tumor size after 12 weeks of therapy. Secondary objectives included assessments of response rate and duration (RECIST), progression-free survival (PFS), and safety. Seventy-one patients were enrolled (cediranib, 53; placebo, 18). The mean percentage change in tumor size between cediranib (−20 %) and placebo (+19 %) was significantly different (p < 0.0001). Eighteen patients (34 %) in cediranib achieved a partial response and 25 patients (47 %) experienced stable disease. Median PFS was longer in cediranib, 12.1 months, vs. placebo, 2.7 months, including placebo group patients who later received cediranib (HR = 0.45; 90 % CI, 0.26–0.78; p = 0.017). The most common adverse events with cediranib were diarrhea (88 %), fatigue (66 %), dysphonia (63 %), and hypertension (61 %) [45]. In this trial 43 patients (81 %) had SD or better. Lastly, cediranib is also being evaluated in a phase II single-arm trial for patients with progressive unresectable, recurrent, or metastatic RCC (NCT00227760).
17.3.7 Tivozanib (AV-951)
Tivozanib (AV-951; AVEO Pharmaceuticals, Inc.) is an inhibitor of VEGF-R-1, VEGF-R-2, and VEGF-R-3 as well as c-Kit and PDGF-R. A phase II study showed that AV-951 was active in RCC with an adverse effect profile consistent with that of a selective VEGF-R inhibitor [46, 47]. This was followed by an open-label phase 3 trial (TIVO-1) comparing tivozanib vs. sorafenib in treatment-naïve or cytokine-pretreated patients with advanced clear cell RCC who had a nephrectomy. While PFS was longer in tivozanib (11.9 months vs. 9.1 months), the overall survival showed a nonsignificant trend toward improved survival in the sorafenib arm (median 29.3 months vs. 28.8 months) [48]. Tivozanib was not approved by the FDA in June 2013 due to inconsistencies in data regarding PFS and OS and an imbalance in post-study treatments, and there are no further development plans in RCC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree