Anatomy and Pathology of the Thyrotrophs
Fabio Rotondo
Kalman Kovacs
Eva Horvath
The anterior pituitary is a highly heterogeneous gland comprised of a variety of cells. Since the late 19th century, considerable progress has been made in understanding the complex physiology of this organ. Although the production of thyroid-stimulating hormone (TSH) or thyrotropin along with the regulatory function of the pituitary gland on the thyroid gland has long been known, it was only approximately over a half century that the adenohypophysial cell type that produces TSH was morphologically identified. In studies of the pituitaries of rats that had altered function of the thyroid and other glands, using periodic acid-Schiff (PAS) and various trichrome, aldehyde fuchsin (AF), and aldehyde thionin (AT) staining techniques, several investigators delineated the thyrotrophs in the rat pituitary anterior lobe (1,2,3,4). Subsequently, the fine structural features of rat thyrotrophs were revealed by transmission electron microscopy, and the ultrastructural alterations resulting from functional changes were disclosed (5,6). Several attempts were also made, through the application of these and other staining procedures, to identify the thyrotrophs in the human pituitary, but it became possible to do this after the introduction of immunohistochemistry and electron microscopy (5).
Development and Anatomy
The anterior pituitary is made up of several distinct cell types that are defined by the hormone they synthesize and secrete. During the developmental stages of the pituitary anterior lobe, the hormone-secreting cell types differentiate in a temporal order and arise in distinct regions (7,8). Thyrotrophs can be detected by immunohistochemistry during the 12th week of gestation in the anteromedial zone of the fetal pituitary (9,10). They become recognizable at about the same time as the gonadotrophs, after the appearance of the somatotrophs and corticotrophs and before that of the lactotrophs. Biologically active TSH has been detected in the pituitary at 14 weeks of gestation although, recent studies confirmed TSH immunoreactivity as early as 9 weeks of gestation indicating that thyrotrophs develop earlier than at 12 weeks of gestation (11). The thyrotrophs also develop in anencephalic fetuses, and tissue culture studies indicate that their differentiation and maturation are independent of the presence of hypothalamic hormones (12,13). A lineage not dependent on the presence of the transcription factor Pit-1 appears first in the rostral part of the gland and disappears by birth. The definitive Pit-1-dependent thyrotrophs appear later in the dorsal region of the gland and persist in the adult (14,15). In rats, mitoses of thyrotrophs contribute to the proliferation of the pituitary gland during the early postnatal period (16,17). Mitosis also has been demonstrated in the thyrotrophs of thyroidectomized adult rats and may be regulated by thyroxine (T4) and thyrotropin-releasing hormone (TRH) (18,19).
Several pituitary transcription factors and other factors have been identified that play important roles in the processes that direct organogenesis, lineage commitment, cell proliferation, and differentiated function. Any or all of these substances are candidates for mutations causing pituitary dysfunction. Signaling gradients of transcription factors formed in a temporal and spatial manner allow distinct cell lineages to arise (20). Transcription factors like Pit-1, GATA-2, and PROP1 play a major role in the differentiation and maturation of thyrotrophs (15,20,21,22,23,24,25,26). These factors also play a role in cell-specific gene expression and regulation of the gene products, namely TSH (15,20,21,27,28). Other factors that may play a role in thyrotroph maturation and function include cleaved prolactin (PRL) variant and neurotensin (NT) (11,29,30,31). The autocrine/paracrine regulation of TSH secretion is evidenced by the pituitary bombesin-like peptide termed neuromedin B, which acts as a local regulator of TSH secretion. It is present in high concentrations in thyrotrophs but its levels can fluctuate according to thyroid status. Neuromedin B is upregulated by thyroid hormones and downregulated by TRH (32,33,34).
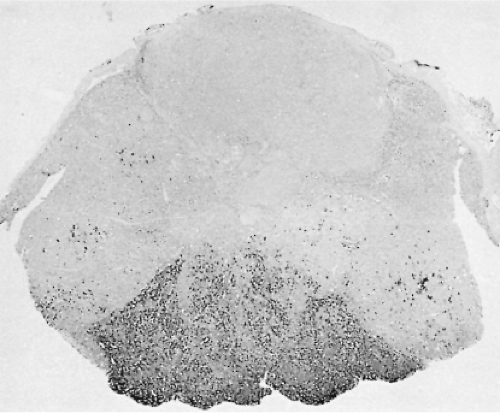
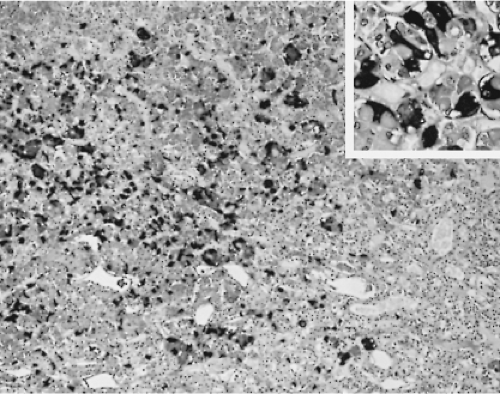
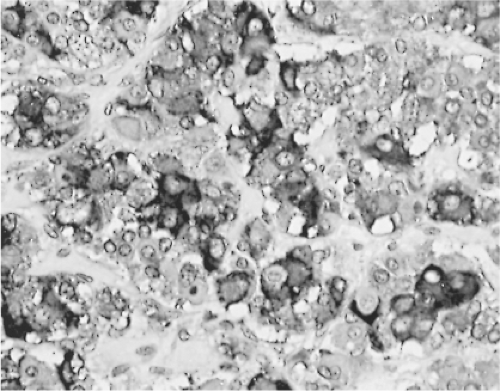
In the human pituitary, the thyrotrophs represent 1% to 5% of the cells in the anterior pituitary gland. They are not randomly located throughout the gland, but are concentrated in the anteromedial portion of the mucoid wedge of the anterior lobe; thus, they are not in proximity to the posterior lobe. In this well-demarcated area, the thyrotrophs are the predominant cell-type (Fig. 3.1) (35). They are chromophobic cells that contain PAS-, AF-, and AT-positive cytoplasmic granules, but they are less basophilic and less PAS-positive than the corticotrophs. They do not stain with acidophilic dyes, lead hematoxylin, erythrosin, or carmoisin. They have an angular appearance with elongated cytoplasmic processes (36). Immunohistochemistry is the most reliable method to reveal the presence of thyrotrophs by light microscopy (Fig. 3.2). In situ hybridization reveals the presence of estrogen receptor mRNA in non-tumorous and adenomatous thyrotrophs (37).
Cytology
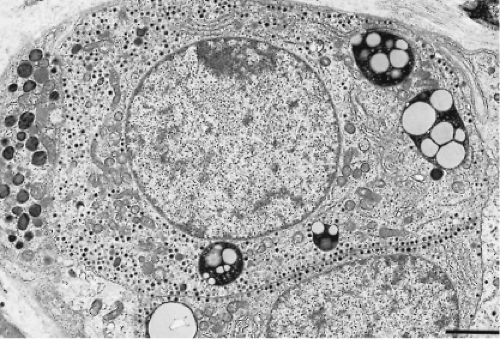
By electron microscopy, the thyrotrophs are medium-sized or large, elongated cells with conspicuous cytoplasmic processes and centrally located eccentric and spherical or ovoid nuclei (Fig. 3.3). The cytoplasm is abundant, and its rough-surfaced endoplasmic reticulum consists of randomly distributed,
slightly dilated cisternae. The Golgi complex is globular and is located in the perinuclear area; its prominence varies depending on the hormonal activity of the cell. The mitochondria are rod shaped with regular transverse cristae and a moderately electron-dense matrix. Phagolysosomes are common. The secretory granules are small, most often measuring 100 to 200 nm. They are spherical, vary slightly in electron density, and are membrane-bound with an electron-lucent halo between the electron-dense core and the limiting membrane. Secretory granule extrusions are not seen.
slightly dilated cisternae. The Golgi complex is globular and is located in the perinuclear area; its prominence varies depending on the hormonal activity of the cell. The mitochondria are rod shaped with regular transverse cristae and a moderately electron-dense matrix. Phagolysosomes are common. The secretory granules are small, most often measuring 100 to 200 nm. They are spherical, vary slightly in electron density, and are membrane-bound with an electron-lucent halo between the electron-dense core and the limiting membrane. Secretory granule extrusions are not seen.
Immunohistochemistry exhibits strong cytoplasmic immunoreactivity for the β-subunit of TSH and for the α-subunit, but not for other hormones (35). Immunoelectron microscopy confirms that the thyrotrophs contain TSH localized in the secretory granules. In the rat pituitary, cells expressing both TSH and growth hormone (GH) were found (38), suggesting a close link between somatotrophs and thyrotrophs, and raising the possibility that somatotrophs can transform to thyrotrophs. Somatotrophs and thyrotrophs share a common precursor cell expressing the transcription factor Pit-l during ontogenesis. Consistent with this suggestion, in the pituitaries of rats made hypothyroid by administration of propylthiouracil, some somatotrophs degranulate and transform into thyroidectomy cells, exhibiting the ultrastructural signs of active secretion (39). Transdifferentiation of somatotrophs to thyrotrophs has also been found in the pituitary of humans with protracted primary hypothyroidism (40,41). These findings support the assumption that different adenohypophysial cell types cannot be conclusively separated and that under special conditions, one cell type may transform to another cell type and become capable of producing the hormone characteristic of the latter cell type. Thus, the “one cell, one hormone” theory that dominated our thinking for several decades, which recognized five distinct cell types producing the six adenohypophysial hormones, is no longer accepted (35,42,43).
Thyrotrophs are present throughout life, and there are no major sex differences in their number, distribution, immunohistochemical profile, and histologic and ultrastructural features. They do not regress in old age, but continue to produce and release TSH (44,45,46). Indeed, the thyrotrophs are more prominent in older people in whom the incidence of subclinical and overt primary hypothyroidism is higher than in young or middle-aged people.
Effect of Thyroid Disorders on Pituitary Morphology
Hypothyroidism
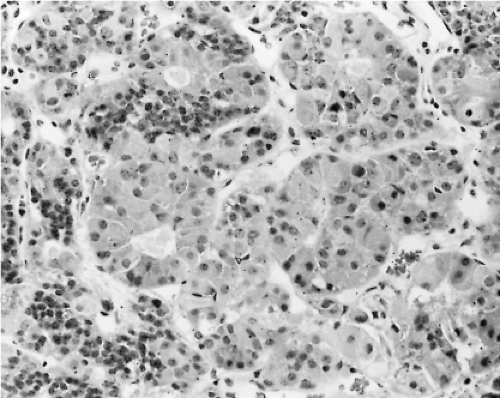
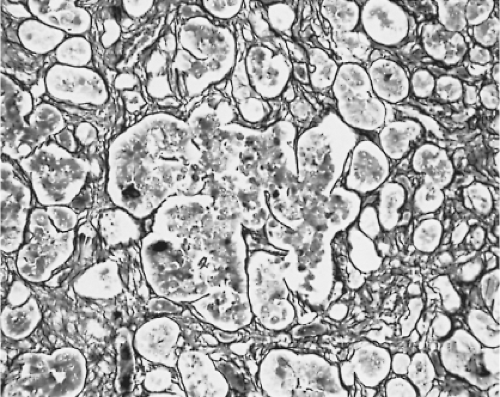
Changes in the secretion of TSH markedly affect the morphology of the thyrotrophs. In patients with long-standing primary hypothyroidism, the thyrotrophs increase in number and size (47) (Figs. 3.4 and 3.5). Reticulin stains show enlarged acinar structures without compression of surrounding tissue
(Fig. 3.6). The large thyrotrophs have an abundant vacuolated cytoplasm and long, prominent cytoplasmic processes, and they contain many large, strongly PAS-positive cytoplasmic globules, which are in fact large lysosomes. These latter structures, however, may occur in other conditions and in other cell types as well (38,48,49).
(Fig. 3.6). The large thyrotrophs have an abundant vacuolated cytoplasm and long, prominent cytoplasmic processes, and they contain many large, strongly PAS-positive cytoplasmic globules, which are in fact large lysosomes. These latter structures, however, may occur in other conditions and in other cell types as well (38,48,49).
Thyrotroph hyperplasia may be diffuse or nodular; the thyrotroph area within the anterior lobe enlarges, and the thyrotrophs extend to other parts of the anterior lobe. The extent of thyrotroph hypertrophy and hyperplasia resulting from hypothyroidism and also altered sensitivity of the pituitary, may be sufficient to cause radiologically detectable enlargement of the pituitary and occasionally even optic nerve compression. Massive thyrotroph hyperplasia due to primary hypothyroidism thus may be difficult to distinguish clinically from a pituitary tumor causing central (secondary) hypothyroidism. Also, several patients with significant pituitary enlargement due to marked thyrotroph hyperplasia have been misdiagnosed as having TSH- or PRL-secreting pituitary adenomas because patients with long-standing primary hypothyroidism may have amenorrhea, galactorrhea, and hyperprolactinemia as well as pituitary enlargement (50,51,52). Hypothyroidism is far more common in women as it is in men. Although hypothyroidism is most common in women who are middle-aged or older, the disease can occur at any age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree