Anatomy and Pathology of Prostate Cancer
▪ 4A Anatomy of the Prostate and the Pathology of Prostate Cancer
Peter A. Humphrey
ANATOMICAL RELATIONSHIPS
The adult prostate gland surrounds the urethra immediately below the base of the urinary bladder and is located posterior to the inferior symphysis pubis, superior to the urogenital diaphragm, and anterior to the rectum (Fig. 4A.1) (1). It has a shape like a truncated cone or a top lying on its side, with the base in direct continuity with the urinary bladder neck. The seminal vesicles lie posterolateral to the base of the bladder and join with the ampulla of the vasa deferentia to form paired intraprostatic ejaculatory ducts. These ejaculatory ducts course through the base of the prostate to their exit at the posterior urethral protuberance, known as the verumontanum (or colliculus seminalis). The utricle, a vestigial müllerian structure, is found as a central depression in the verumontanum. The prostatic urethra is formed at the bladder neck, turns anteriorly 35° at its midpoint (the urethral angle), and exits the prostate at its apex, where it is continuous with the membranous urethra. The urethral angle divides the urethra into proximal (the so-called preprostatic) and distal (the so-called prostatic) segments. The apex of the prostate is attached to the urogenital diaphragm. Finally, a patch of nonglandular tissue called the anterior fibromuscular stroma is present anteriorly over the prostate and extends from the bladder neck to the apex of the prostate. The prostate measures 5 × 4 × 3 cm and weighs 20 g from ages 20 to 50, with an increase to 30 g from age 60 to 80 (1).
ANATOMICAL ZONAL MODELS
Over the past century, anatomical models of the prostate have emphasized the concepts of lobes or zones of the prostate (1,2,3). It had been proposed that the prostate possessed anywhere from two to six lobes. In the first half of the 20th century, the five-lobe model dominated, but this work was based on embryonic and fetal prostates, not adult glands. In 1954, Franks put forth the idea of an outer gland (where carcinomas arose) and a periurethral inner gland (3). Further evolution of this concept occurred when McNeal developed the zonal model that is currently widely used (1,2). This model defines three zones: a central zone, a transition zone, and a peripheral zone (Fig. 4A.1). It can be difficult to grossly and microscopically recognize a sharp demarcation between these zones, although occasionally, a fibrous band separating the transition zone from the peripheral zone can be seen at the macroscopic level. Of importance, these zones do appear to have structural differences, and diseases differentially affect these zones. Carcinoma preferentially affects the peripheral zone, benign prostatic hyperplasia (BPH) is typically found in the transition zone, and the central zone is remarkably resistant to disease. (See below section on location of prostate cancer).
The transition zone, which normally makes up 5% of prostate gland volume, wraps around the proximal urethra. The main ducts of this zone drain into posterolateral recesses of the urethra at a point just proximal to the urethral angle.
Some of the more medial ducts penetrate through the thick smooth muscle bundles of preprostatic sphincter. Also found in the smooth muscle of the preprostatic sphincter are minute ducts and abortive acinar arrangements of periurethral glands, which constitute only about 1% of the total glandular volume of the prostate. The central zone makes up about 25% of the entire prostate gland and is a posteriorly situated cone-like structure with the base of cone projecting toward the base of the bladder. The paired ejaculatory ducts run through the central zone. The ducts from the central zone drain into the urethra via orifices positioned just next to the ejaculatory duct orifices on the verumontanum. The peripheral zone of the prostate gland is the bulk of the posterior, lateral, and apical portion of the prostate gland and accounts for 70% of the total gland volume. The ducts of this zone empty into the posterior urethral recesses or grooves in a double row from the verumontanum to the prostatic apex.
Some of the more medial ducts penetrate through the thick smooth muscle bundles of preprostatic sphincter. Also found in the smooth muscle of the preprostatic sphincter are minute ducts and abortive acinar arrangements of periurethral glands, which constitute only about 1% of the total glandular volume of the prostate. The central zone makes up about 25% of the entire prostate gland and is a posteriorly situated cone-like structure with the base of cone projecting toward the base of the bladder. The paired ejaculatory ducts run through the central zone. The ducts from the central zone drain into the urethra via orifices positioned just next to the ejaculatory duct orifices on the verumontanum. The peripheral zone of the prostate gland is the bulk of the posterior, lateral, and apical portion of the prostate gland and accounts for 70% of the total gland volume. The ducts of this zone empty into the posterior urethral recesses or grooves in a double row from the verumontanum to the prostatic apex.
A patch of nonglandular tissue, the anterior fibromuscular stroma, is located anteriorly over the prostate and extends from the bladder neck to the apex of the prostate (4). The anterior fibromuscular stroma is composed of fibrous tissue and compact smooth muscle bundles that fuse with smooth muscle tissue of the bladder neck at the prostate base and that interdigitate with skeletal muscle of the urogenital diaphragm at the apex. The external surface of the anterior fibromuscular stroma is in continuity with adipose tissue in the space of Retzius (1).
Additional nonglandular tissues are the preprostatic sphincter and the striated sphincter (1). The preprostatic sphincter is a cylinder of smooth muscle that encircles the proximal segment of the prostatic urethra. The striated sphincter is comprised of skeletal muscle fibers found around the distal prostatic urethra, between the verumontanum and the prostatic apex. It is incomplete posterolaterally and is contiguous with the external urethral sphincter distal to the prostatic apex.
OUTER “CAPSULE”
The outer prostatic “capsule” is actually a band of concentrically placed fibromuscular and vascular tissue that is inseparable from prostatic stroma and surrounding fasciae (1,5). Outside the prostate, this band is also continuous with pelvic fascia and rectovesical fascia (of Denonvillier). Denonvilliers fascia is a sheath between the rectum and prostate that covers the posterior aspect of the prostate and seminal vesicles. The fibromuscular band (“capsule”) of the prostate is 0.5 to 3 mm thick and is incomplete, being absent at the apex (5). It is composed mainly of transversely disposed bundles of smooth muscle and collagen. Smooth muscle bundles course between the band and periepithelial stroma inside the prostate gland, without obeying any sort of boundary. Also, the concentration of smooth muscle fibers in the band and prostatic stroma is identical. Glands from the prostate approach this band, but the border between the outer limit of epithelium and the band is not often clearly definable under the light microscope. Posteriorly, the band fuses with Denonvilliers fascia and does not clearly separate prostate from seminal vesicle. Anteriorly and anterolaterally, the band blends with the pelvic fascia. Apically, the band is no longer present, and instead there is a jumble of smooth muscle, skeletal muscle, and fibroelastic fibers. Here, it is important to know that the urethral striated sphincter (rhabdosphincter) is in direct continuity with the prostate, and indeed skeletal muscle fibers can normally be found, at an anterior and apical location, within the substance of the gland itself. At the bladder neck, a capsular separation of bladder and prostate also does not exist; rather, there is a fusion of smooth muscle bundles. As there is no true histologically distinct prostatic “capsule,” it is preferable not to refer to “capsular penetration or extracapsular extension” when noting tumor that has spread beyond the prostate. Rather, a more appropriate designation is to state whether tumor is organ-confined or shows extraprostatic extension.
“SURGICAL CAPSULE”
The term “surgical capsule” has been used to refer to an intraprostatic surgical plane of dissection when enucleating nodules of transition zone BPH by blunt dissection between the nodules and the peripheral zone. However, histologically, a true capsule (a discrete fibrous or fibromuscular band of tissue) does not separate BPH nodules from the peripheral zone.
BLOOD SUPPLY AND LYMPHATIC DRAINAGE
The blood supply to the prostate is from the inferior vesical artery, which branches off the internal iliac artery and which terminates in capsular and urethral arterial groups. Venous drainage is into the plexus of Santorini.
There is a network of intraprostatic lymphatics that drain principally into obturator and then internal iliac lymph nodes. A minor amount of drainage occurs through the presacral group and external iliac nodes. An uncommon site of flow is through periprostatic or periseminal vesicle lymph nodes, which are found in about 4% of radical prostatectomy specimens (6). These tend to be small lymph nodes, averaging about 2 mm in greatest dimension.
NERVES
The prostate gland has a rich nerve supply, with sympathetic and parasympathetic innervation arising from the pelvic plexus (7). These nerves run with branches of the capsular artery and penetrate the prostate, where parasympathetic fibers course to acini and stimulate secretion and where sympathetic fibers cause contraction of outer band “capsular” and intraprostatic smooth muscle. Innervation of the peripheral zone and posterior “capsule” is significantly higher than that of the transition zone and anterior “capsule” (7). Histologically, nerves are seen in the periprostatic neurovascular bundle, in the outer fibromuscular band, and in the prostate itself. Ganglia and paraganglia are usually found outside the gland but may also be found in the outer fibromuscular band and within the prostate. Paraganglia are most often located in or adjacent to the lateral neurovascular bundles. It should be noted that benign prostatic glands can abut nerves (8) and can even be found within nerves and this should not be considered perineural invasion by carcinoma.
NORMAL PROSTATE HISTOLOGY AND IMMUNOPHENOTYPE
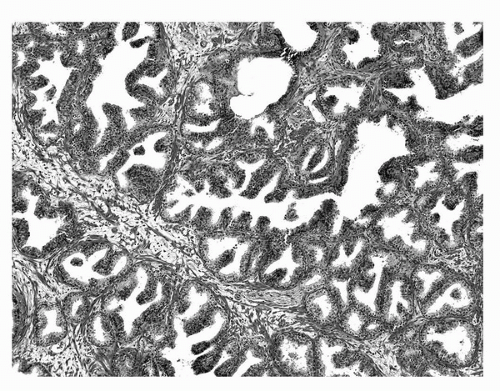
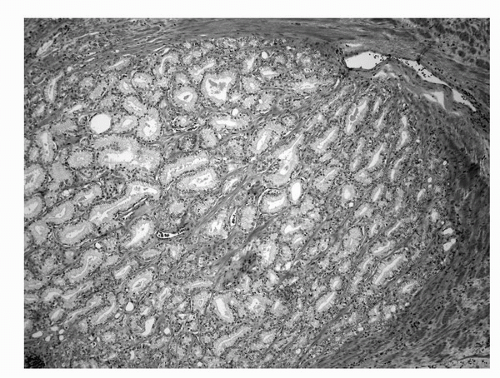
Microscopically, “normal” adult prostate tissue is best visualized in the prostate glands of men 20 to 50 years of age, when significant distortion by inflammation, atrophy, and hyperplasia has not developed. The normal adult prostate is a branching duct-acinar glandular system embedded in a dense fibromuscular stroma (Fig. 4A.2). Zonal architectural differences can be appreciated (1). Normally, the peripheral zone ducts and acini are evenly distributed but are irregular in size and shape. Normal transition zone glands are similar
to those of the peripheral zone. Central zone glands are more densely arranged than peripheral and transition zone glands. Also, central zone glands are larger and display intraluminal projections with fibrovascular cores. Central zone epithelium displays tall columnar cells with eosinophilic cytoplasm, a prominent basal cell layer, and on occasion complex intraluminal architecture such as Roman bridge and cribriform formations. These findings can be misdiagnosed as atypia or prostatic intraepithelial neoplasia (PIN) (9).
to those of the peripheral zone. Central zone glands are more densely arranged than peripheral and transition zone glands. Also, central zone glands are larger and display intraluminal projections with fibrovascular cores. Central zone epithelium displays tall columnar cells with eosinophilic cytoplasm, a prominent basal cell layer, and on occasion complex intraluminal architecture such as Roman bridge and cribriform formations. These findings can be misdiagnosed as atypia or prostatic intraepithelial neoplasia (PIN) (9).
The normal epithelium of the adult prostate is classically defined as having two cell layers: a luminal or secretory cell layer and a basal cell layer (Fig. 4A.3). A third cell type in normal prostatic epithelium is the neuroendocrine cell, which is rare. The secretory or luminal cells of the normal prostate glandular epithelium make up the bulk (about three-quarters) of the epithelial volume. The secretory cells are cuboidal to columnar with nuclei positioned in the basal to midportion of the cell. Cleared cytoplasm is a hallmark of normal prostatic secretory cells due to the presence of a large number of small clear secretory vacuoles. The central zone secretory cell cytoplasm is somewhat denser because it contains a smaller number of these vacuoles (1). The nuclei of normal prostatic secretory epithelial cells are small and round with fine, evenly dispersed chromatin. Nucleoli usually are not evident or are pinpoint in size. Nuclei in the central zone usually are larger than those in the peripheral zone and also appear crowded. Normal secretory cells are immunoreactive for pan-cytokeratins, cytokeratins 8 and 18, prostate-specific antigen (PSA), prostatic-specific acid phosphatase (PSAP), and the androgen receptor.
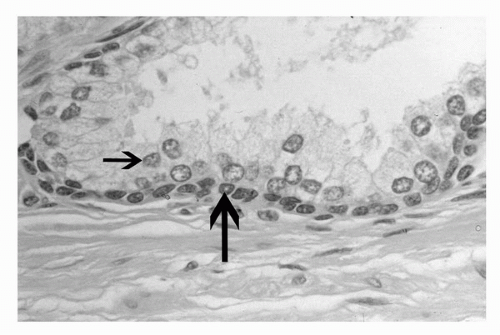 FIGURE 4A.3. Normal prostate gland with luminal secretory cells (small arrow) and underlying basal cells (large arrow). |
The basal cell layer separates the secretory cells from the basement membrane and is nearly continuous. Basal cells in the prostatic epithelium often appear as rounded or oblong cells, but can also be flattened, spindled, cuboidal, and triangular (10). Basal cells have a scant amount of dense cytoplasm and small, hyperchromatic nuclei. A stem cell population may reside within basal cells in the prostate (11). The immunophenotype of basal cells is distinctive, is different from luminal secretory cells, and can be of diagnostic value, since basal cell loss is characteristic of adenocarcinoma of the prostate. (See below section on immunohistochemistry).
Neuroendocrine (endocrine-paracrine) cells constitute a third population of cells in normal prostatic epithelium (12). These cells are a small minority at about 0.4% of the total adult prostatic epithelial cell population. This is a terminally differentiated, postmitotic cell population. These neuroendocrine cells are usually recognizable only by histochemical (argentaffin or argyrophil) stains or immunohistochemical staining (e.g., for chromogranin). Normal neuroendocrine cells variably express androgen receptor, a variety of neuropeptides, and the secretory products PSA and PSAP. The keratin expression pattern is more like luminal than basal cells.
Urothelium is another type of epithelial cell normally found in the prostate. In addition to lining the prostatic urethra, urothelium also normally lines major prostatic ducts. This ductal lining is variable in extent. The urothelium in the prostatic ducts differs from such lining elsewhere in the urinary tract in that an umbrella layer of cap cells is lacking and is replaced by secretory cells. Urothelium is a multilayered epithelium with rounded to elongated nuclei, some of which possess nuclear grooves. A degree of cytoplasmic clearing usually is evident.
Intraluminal contents of normal prostatic glands include shed and degenerating epithelial cells, corpora amylacea, and calculi. Corpora amylacea are inspissated secretions that often assume a concentrically lamellated appearance like rings in a tree. They often calcify and probably contribute to the formation of prostatic calculi. Prostatic calculi are also extremely common, being detectable in 75% to 100% of men by ultrasonography (13). They are typically found in central, large prostatic ducts. These stones vary in size from microscopic to 4 cm in size and grossly are usually multiple, round or ovoid, and brown, with variable white or gray areas. Prostatic stones mainly contain calcium phosphate, but calcium oxalate, carbonate-apatite, and hydroxyapatite may also be present. Prostatic calculi are most often seen as incidental findings in the setting of inflammation and BPH. The diagnostic impact of corpora, microcalcifications, and calculi is that they are all more common in benign glands, but their presence does not rule out malignancy.
The stroma in the prostate gland consists chiefly of smooth muscle cells and fibroblasts, with ramifications of blood and lymph vessels as well as nerve bundles and axons (1). The stroma of the transition zone is more compact than that of the peripheral zone, with more densely concentrated smooth muscle bundles. As noted above, skeletal muscle fibers can be found admixed with smooth muscle stroma and normal prostate glands at the distal apex of the prostate. Normal and benign glands in this skeletal muscle tissue should not be mistaken as neoplastic. The extracellular stromal matrix of the prostate is primarily collagen of types I and III, complex polysaccharides, and glycosaminoglycans such as dermatan sulfate, heparin, chondroitin, and hyaluronic acid (1). Fibronectin and tenascin polypeptides are also found in prostatic stroma. Abutting prostatic glands is a delicate basement membrane that, at 100 nm in thickness, is too small to be seen in hematoxylin and eosin-stained sections. Rarely to uncommonly, adipocytes can be seen in the prostate (14). For practical purposes,
carcinoma invading fat should be considered to represent extraprostatic extension.
carcinoma invading fat should be considered to represent extraprostatic extension.
PATHOLOGY OF PROSTATE CANCER
Gross Diagnosis
Macroscopic identification of prostatic carcinoma in needle biopsy tissue and transurethral resection of prostate (TURP) chips is generally not possible.
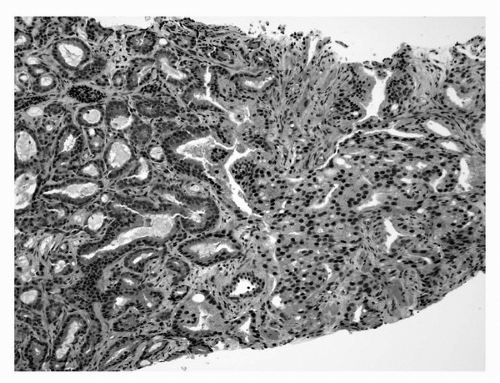
Grossly it can be difficult or impossible to recognize prostatic carcinoma, even with the whole gland available for evaluation (15). Palpation of the excised, intact prostate gland can be helpful in a minority of cases in delineating the site(s) of nodularity, but in this era when most carcinomas are clinically impalpable, they are also impalpable at the surgical pathology cutting bench (15). When visible on cut sections, carcinoma can appear nodular and white (Fig. 4A.4) to irregular gray or white-yellow compared with adjacent benign prostatic tissue, which is tan, spongy, and often cystic. Macroscopically recognizable carcinoma tends to be, not surprisingly, of higher grade, stage, and size (15). The lower limit of size detection of gross carcinoma is about 0.5 cm. More often, no gross lesion is evident or a lesion only suggestive of carcinoma is visualized (15). Subtle gross changes in cut sections are best appreciated by comparing right and left halves of the prostate with each other. These subtle alterations include ill-defined, asymmetric, firm, yellow-white areas in the peripheral zone. The more centrally located transition zone carcinomas are characteristically nodular, solid, and bright yellow to white (16), but BPH nodules can precisely simulate this appearance. Attempts to recognize gross extension of carcinoma into extraprostatic tissues and surgical margins in radical prostatectomy specimens can also be a futile exercise.
Location
Most adenocarcinomas arise in the peripheral zone, where posterolateral or posterior positions are typical, with anterior peripheral zone carcinomas seen in only about 7% of cases (17). The predominant carcinoma is in the peripheral zone in about 70% of cases, in the transition zone in about 20% of cases (18,19), and in the central zone in about 5% of cases (20). In roughly one quarter of cases, cancer foci are identified in both the transition and peripheral zones (19).
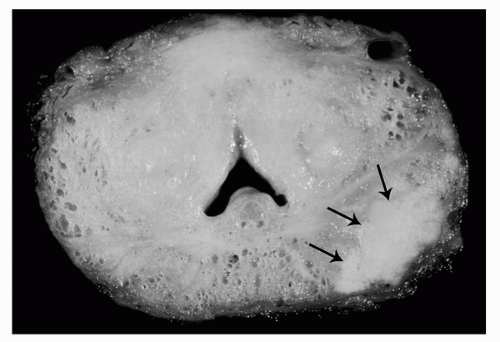 FIGURE 4A.4. Gross appearance of prostatic carcinoma in radical prostatectomy specimen: solid white posterolateral peripheral zone nodule (arrows). |
There are clinical and histopathological differences between transition zone and peripheral zone carcinomas (19,21). Transition zone cancers are more likely to be detected as an incidental finding in TURP chips since TUR selectively samples the transition zone. Transition zone carcinomas tend to lower Gleason grade (with a mean score of 5) compared to peripheral zone carcinomas (with a mean score of 6-7), and transition zone carcinomas less often exhibit extraprostatic extension and seminal vesicle invasion. However, a subset of transition zone carcinoma is high-grade with Gleason grade 4 or 5, with high rates of extraprostatic extension (22). Because of their location transition zone carcinomas often invade into anterior fibromuscular stroma and BPH nodules, while those peripheral zone carcinomas that exhibit extraprostatic extension often invade into posterolateral periprostatic soft tissue. Some transition zone carcinomas can be high-volume, and low-grade with a high preoperative PSA and still organ-confined (23). Histologically, it has been previously proposed that transition zone carcinomas have a distinctive histologic appearance, with columnar cells lining glands of variable shape and contour (18), but these features are not specific and can be seen in a substantial percentage of peripheral zone carcinomas (24). There are some differences between transition zone and peripheral zone carcinomas in DNA ploidy, proliferation index, microvessel density, and p53, bcl2, and matrix metalloproteinase expression as well as molecular genetic differences (19), but these could be related to Gleason grade differences. The outcome data after radical prostatectomy are mixed: some studies show a better outcome for transition zone compared to peripheral zone cancers (21,25), whereas others show no difference (26). It does not appear that transition zone versus peripheral zone location of prostate cancer is an independent prognosticator. Moreover, it is difficult to sample transition zone carcinoma by needle biopsy. Needle biopsies directed toward the transition zone do not adequately sample clinically relevant transition zone carcinomas, since the biopsies often did not sample the transition zone or the dominant transition zone carcinoma (27).
Central zone carcinomas are uncommon, representing 3% to 8% of all prostate cancers (20,28). In one series, these carcinomas were of high grade (with a mean Gleason score of 8) with high rates of extraprostatic extension, seminal vesicle invasion, and biochemical failure after radical prostatectomy (28). Central zone location is not an independent predictor of clinical failure.
Multifocality
Prostatic adenocarcinomas are multifocal in 50% to 97% of cases (28,30). There is histologic (Gleason grade) and molecular heterogeneity of multifocal adenocarcinoma within the same prostate gland (30,31,32,33). Comparative genomic hybridization and TMPRSS2 gene rearrangement studies suggest that multifocal prostatic carcinomas arise from multiple, independent clonal expansions (32,33).
Histologic Diagnosis of Adenocarcinoma in Prostate Needle Biopsy Tissue
The definitive diagnosis of prostatic carcinoma is most often established by light microscopic interpretation of hematoxylin and eosin-stained sections of needle core biopsy tissue procured
from the peripheral zone. The histologic diagnosis of prostatic carcinoma requires a synthesis of a constellation of features that allows for a definitive diagnosis. A conceptual framework for a rationale approach to this diagnosis entails application of major and minor criteria (34). The criteria are utilized in all prostatic tissue samples. To follow is a description of this approach in needle biopsy.
from the peripheral zone. The histologic diagnosis of prostatic carcinoma requires a synthesis of a constellation of features that allows for a definitive diagnosis. A conceptual framework for a rationale approach to this diagnosis entails application of major and minor criteria (34). The criteria are utilized in all prostatic tissue samples. To follow is a description of this approach in needle biopsy.
The major criteria are an infiltrative growth pattern, absence of basal cells, and nuclear atypia. The first of the major criteria, an infiltrative growth pattern, frequently presents as the presence of small crowded malignant glands extending between and/or around larger, more complex, (and often paler) benign glands. These Gleason pattern 3 adenocarcinomas represent the most common pattern recognized in needle biopsy either in pure form as Gleason score 6 (3 + 3) (Fig. 4A.5) or as Gleason score 7, admixed with Gleason pattern 4 (Fig. 4A.6) (35,36,37). (See below section on Gleason grading). Gleason pattern 3 shows haphazardly arranged and variably sized individual, discrete glands (37). The infiltrative character of high-grade (Gleason patterns 4 or 5 and score 7-10) prostatic carcinoma in needle biopsy is typified by ragged invasion of fused microacinar, cribriform, or papillary masses (Fig. 4A.7). Linkage of carcinoma cells into chains and cords also indicates poorly differentiated Gleason pattern 4 adenocarcinoma. Another descriptor of Gleason pattern 4 is ill-defined glands with poorly formed glandular lumina (37). High-grade carcinoma can also invade as sheets, with destruction and effacement of benign prostatic tissue (Fig. 4A.8), or be manifested as comedocarcinoma. These arrangements represent Gleason pattern 5, which is uncommon in needle biopsy (36).
Uncommonly, invasion by carcinoma into extraprostatic tissues sampled by needle biopsy can be appreciated. As incidental findings, periprostatic tissue can be found in up to 77% of needle biopsies (38) and seminal vesicle or ejaculatory duct tissue can be seen in up to 20% of needle biopsies (39). Periprostatic adipose tissue is most often seen at the tips of the needle cores and the presence of carcinoma in this fat can be a useful diagnostic finding. However, this is rarely an isolated finding, and is generally associated with extensive carcinoma in the rest of the needle biopsy tissue. Prostatic carcinoma infiltration into seminal vesicle/ejaculatory duct tissue is an uncommon, incidental discovery, which tends to be more common in extensive, higher grade carcinoma in needle biopsy. Thus, in prostatic needle biopsy, carcinoma of the prostate can present a range of images of infiltration, with invasion into prostatic as well as extraprostatic tissues.
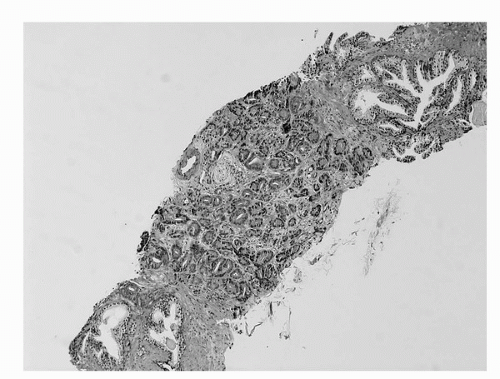 FIGURE 4A.5. Adenocarcinoma, Gleason grade 3 + 3 = score of 6 in needle core tissue. Single separate small acini infiltrate across width of needle core. |
 FIGURE 4A.7. Adenocarcinoma, Gleason grade 4 + 4 = score of 8 in needle core tissue: masses of fused glands. |
In a small minority of needle biopsy cases, an infiltrative pattern is not evident. These appear to be well-differentiated, Gleason score 3 to 4, adenocarcinomas that are, by definition, well-circumscribed small gland proliferations, with smooth, pushing edges (35,37). However, an entire Gleason score 3 to 4 nodule is not captured by needle biopsy and also, well-differentiated Gleason score 3 and 4 nodules are characteristically found in the transition zone of the prostate, whereas it is the peripheral zone that is typically targeted for needle biopsy. Most apparently well-differentiated adenocarcinomas in needle biopsy tissue actually represent intermediate-grade carcinomas at follow-up radical prostatectomy (35), such that Gleason score 3 to 4 should be rarely, if ever, assigned to adenocarcinoma in needle biopsy tissue (37).
The second of the major criteria for diagnosis of adenocarcinoma is absence of basal cells in the atypical glands. A historic challenge for the surgical pathologist has been the distinction of periglandular stromal fibroblasts from basal cells. Another common difficulty is that distorted, crushed, or poorly preserved carcinoma cells in minimal cancer foci can mimic basal cells. In cases such as these, application of a basal cell-specific immunohistochemical stain for high-molecular-weight
cytokeratin and/or p63 can be diagnostically advantageous. (See below section on immunohistochemistry). However, these immunostains are not “malignant stains,” and basal cells can be lost, usually in fragmented fashion, in benign glands. Specifically, basal cells may be completely absent in scattered benign and especially atrophic glands and a fragmented basal cell layer is characteristic of atypical adenomatous hyperplasia (adenosis) where, on average, 50% of glands lack a basal cell layer (40). Additional but rare benign mimickers of prostatic carcinoma that can lack basal cells include mesonephric hyperplasia and nephrogenic adenoma. Loss of basal cells in a few glands of partial atrophy or crowded benign glands is a common problem that leads to diagnostic difficulty (41). To emphasize, absence of basal cells is a central finding in an atypical gland proliferation, but by itself, it is not fully diagnostic of adenocarcinoma (42).
cytokeratin and/or p63 can be diagnostically advantageous. (See below section on immunohistochemistry). However, these immunostains are not “malignant stains,” and basal cells can be lost, usually in fragmented fashion, in benign glands. Specifically, basal cells may be completely absent in scattered benign and especially atrophic glands and a fragmented basal cell layer is characteristic of atypical adenomatous hyperplasia (adenosis) where, on average, 50% of glands lack a basal cell layer (40). Additional but rare benign mimickers of prostatic carcinoma that can lack basal cells include mesonephric hyperplasia and nephrogenic adenoma. Loss of basal cells in a few glands of partial atrophy or crowded benign glands is a common problem that leads to diagnostic difficulty (41). To emphasize, absence of basal cells is a central finding in an atypical gland proliferation, but by itself, it is not fully diagnostic of adenocarcinoma (42).
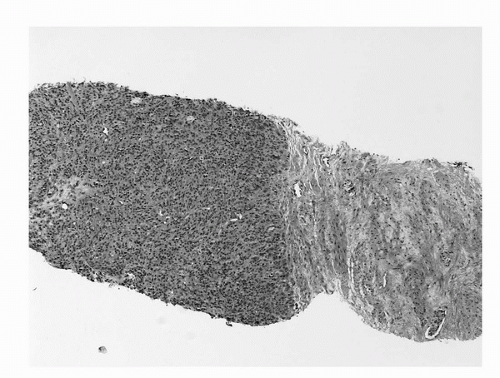 FIGURE 4A.8. Adenocarcinoma, Gleason grade 5 + 5 = score of 10 in needle core tissue: sheet-like growth is present. Benign stroma is present at right. |
Nuclear atypia in the form of nuclear enlargement and nucleolar enlargement is the third of the major criteria for diagnosis of adenocarcinoma. Nuclear atypia in malignant glands most often manifests itself as nuclear enlargement and prominent nucleoli. Prominent nucleoli, however, are neither absolutely specific nor sensitive in detection of adenocarcinoma nuclei. For example, nuclei in inflamed benign prostatic epithelial luminal cells or basal cells can harbor large nucleoli. Some prostate cancers do not harbor macronucleoli. Examples include foamy gland carcinoma, which can have shrunken nuclei, prostatic carcinoma with androgen deprivation therapy effect, and some well-differentiated Gleason score 2 to 4 adenocarcinomas (the so-called nucleolus-poor adenocarcinomas). The latter two examples are usually not seen in needle biopsy tissue. One essential point is that when macronucleoli are absent, there should be significant nucleomegaly with or without nuclear hyperchromasia to definitively diagnose carcinoma.
The minor diagnostic criteria include intraluminal mucin, pink amorphous secretions, mitotic figures, intraluminal crystalloids, and amphophilic cytoplasm. These minor or “soft” diagnostic findings are not specific for carcinoma but are useful for prompting in-depth study of the glands harboring these changes, with a view toward assessment of the aforementioned major diagnostic criteria.
There are several histologic features that have been forwarded as specific for malignancy (43)—perineural invasion, collagenous micronodules (also known as mucinous fibroplasia), and glomeruloid intraglandular projections. True perineural invasion, which is characteristic of adenocarcinoma of the prostate, needs to be distinguished from benign glands abutting prostatic peripheral nerve. Collagenous micronodules are a distinctive, but uncommon, type of stromal response to invasive prostatic carcinoma, which often exhibits mucinous differentiation. Adenocarcinomas of the prostate with glomeruloid features are remarkable for intraglandular tufts of malignant cells that resemble (somewhat) renal glomeruli in their low-power appearance. Another finding specific for carcinoma—lymphvascular space invasion—is vanishingly rare in prostate needle biopsy tissue.
Minimal (Limited) Adenocarcinoma in Prostate Needle Biopsy Tissue
One of the major diagnostic challenges confronting the histopathologist in prostate needle biopsy interpretation is the definite establishment of a malignant diagnosis based on a minimal or limited amount of carcinoma in needle biopsy, where there are just a few malignant glands present (44,45). Minimal carcinoma in prostate needle core tissue is often defined as carcinoma of <1 mm in length or involving <5% of needle core tissue (Fig. 4A.9). The major and minor diagnostic criteria should be applied here, just as for more extensive carcinoma. The presence of a few small malignant glands located between larger, more complex, paler benign glands is the most common pattern of invasion in minimal carcinoma, accounting for fully 80% of cases in one series (45). The second most common pattern of infiltration of limited carcinoma is a haphazard growth in stroma, without adjacent benign glands. Uncommon patterns of growth that typically accompany the common patterns are cords of cells, single cells, and cribriform glands. Perineural invasion is distinctly uncommon (at 2%) in cases of minimal carcinoma (44).
The major criteria do not include a quantitative threshold for the number of glands required to establish a diagnosis of malignancy. In one series (44), 80% of minimal carcinoma foci contained more than 10 glands, and in a second series (45), the median number of malignant glands was 20. It is possible to diagnose invasive carcinoma based on just a few glands. For most urologic pathologists, the smallest number of atypical glands, which formed the basis for a definitive diagnosis of malignancy, was three to four (34). In these cases, there should be excellent preservation of cellular detail, without tangential sectioning, such that it is clear on the H&E sections that basal cells are absent and that significant nuclear atypia is present. Both of these major criteria are of immense importance for these most minimal of the minimal carcinomas, where architectural
abnormalities are difficult to impossible to discern. Finally, in this era, there is a trend to confirm the diagnosis of minimal adenocarcinoma with immunohistochemical stains (42).
abnormalities are difficult to impossible to discern. Finally, in this era, there is a trend to confirm the diagnosis of minimal adenocarcinoma with immunohistochemical stains (42).
 FIGURE 4A.9. Minimal adenocarcinoma, Gleason grade 3 + 4 = score of 7 in needle core tissue, measuring <1 mm in length. |
A small or minimal amount of cancer in prostate needle core tissue does not always equate with a small amount of carcinoma in the whole gland (46). The correlation of amount of cancer in needle core tissue with amount of cancer in the whole gland is not high. One can have greater confidence of the presence of a larger cancer in the whole gland if there is extensive core involvement compared to attempts to predict small cancers in the whole gland based on a small or minimal amount of cancer in needle core tissue. Of importance, minimal or limited carcinoma in needle core tissue does not equate with well-differentiated Gleason score 2 to 4 adenocarcinoma. Most minimal adenocarcinomas are Gleason score 6, but minimal high-grade Gleason score 7 to 10 adenocarcinomas can also be diagnosed in a minority of patients (44,45).
Differential Diagnosis of Adenocarcinoma in Prostate Needle Biopsy
Numerous entities, both benign and malignant, should be considered in the histologic differential diagnosis of prostatic adenocarcinoma. In-depth discussion of all these entities is beyond the scope of this chapter. The next sections highlight the differential diagnostic considerations of focal glandular atypia, also known as atypical small acinar proliferation (ASAP), false-positive diagnoses, false-negative diagnoses, and urothelial carcinoma in the prostate.
Focal Atypia or Atypical Small Acinar Proliferation
A descriptive diagnosis that may be rendered if the histological and/or immunohistochemical findings are felt to be worrisome, but not fully diagnostic of carcinoma, is atypia (47) also known as ASAP. Such a diagnosis is rendered in about 4% to 5% of all prostate needle biopsy cases (47).
This is a descriptive diagnosis for a gland or group of glands with architectural or cytological atypia that does not allow for a definitive diagnosis of reactive atypia, adenosis, PIN, or carcinoma. Distortion artifact, section thickness, and overstaining can contribute to difficulty in interpretation. Immunohistochemistry can be very useful in addressing the differential diagnosis of atypia versus minimal adenocarcinoma and has been shown to reduce the rate of atypia diagnoses. Patients with a diagnosis of atypia (ASAP) should be clinically followed and re-biopsied because about 43% of men are diagnosed with carcinoma on re-biopsy (47).
False-Positive Histologic Diagnoses of Adenocarcinoma in Needle Biopsy Tissue
There are numerous benign histologic mimickers of prostate cancer in prostate needle biopsy tissue (48,49) that can be misdiagnosed as malignant. The rate of false-positive diagnoses in needle biopsy cases is around 1% (50). False-positive diagnoses have also been documented in TURP chips (51). The benign entities most likely to be misdiagnosed as carcinoma in needle biopsy include atrophy, adenosis (atypical adenomatous hyperplasia), and crowded benign glands (48,49,50).
Atrophy of prostatic glands is the benign condition most likely to be misdiagnosed as prostatic carcinoma by light microscopy. It is a common, age-related process that could be related to inflammation, hormones, obstruction, or ischemia. It can also be caused by treatment, including radiotherapy and hormonal therapy. Proliferative inflammatory atrophy has been forwarded as a potential precursor for prostatic intraepithelial neoplasia and thereby prostate cancer (52,53). However, atrophy detected in needle biopsy tissue does not appear to be a risk factor for subsequent detection of carcinoma (54) and therefore does not need to be reported.
Atrophy is common in needle biopsy tissues. Histologically, atrophy is defined as cytoplasmic volume loss. It is not necessary to subtype atrophy, but it is important to recognize the existence of different histomorphological patterns including simple atrophy (with or without cystic change), sclerotic atrophy, partial atrophy, and postatrophic hyperplasia (or hyperplastic atrophy). Atrophy can be confused with carcinoma because it is usually a small gland lesion that can show a pseudoinfiltrative pattern of growth, stromal sclerosis, nuclear atypia, and closely packed acini (in postatrophic hyperplasia). Another diagnostic pitfall is that atrophic glands can show a fragmented basal cell layer and even complete loss of basal cells in a few glands, using immunohistochemical stains for basal cells (such as 34βE12 and p63) (42). Also, the selective but not specific marker for neoplastic epithelial cells, alphamethylacyl CoA racemase (AMACR), can be focally positive in atrophy by immunostaining.
Adenosis, also known as atypical adenomatous hyperplasia, is a nodular proliferation of closely packed small acini. It is invariably an incidental histologic finding, most often found in transition zone tissue in TURP chips or prostatectomy specimens, although it can be seen in about 1% of needle biopsy cases. The densely packed small pale acini are sometimes intermingled with larger more complex glands. Nuclear atypia is absent to minimal. The basal cell layer is fragmented, and on average 50% of glands completely lack basal cells. Of note, AMACR is diffusely positive in about 8% of cases, which can be mistaken as an indictor of malignancy. Adenosis can be mistaken for well-differentiated Gleason score 2 to 4 adenocarcinoma. It does not have known premalignant potential.
Additional benign glandular changes less commonly misdiagnosed as malignant include radiation-induced atypia in benign glands, inflammatory atypia, and basal cell hyperplasia. Immunohistochemical stains can help resolve some difficult diagnostic cases, but do not always provide definite proof of benignancy versus malignancy.
False-Negative Histologic Diagnoses of Adenocarcinoma in Needle Biopsy Tissue
The incidence of a false-negative diagnosis of adenocarcinoma in prostate needle biopsy tissue is also around 1% (55). The carcinomas that are likely to be missed are minimal (<1 mm in length), with variant architectural and/or nuclear features that cause difficulty in diagnostic recognition on standard H&E-stained slides. These deceptively benign-appearing histological variants include pseudohyperplastic pattern (mimicking benign glandular hyperplasia), atrophic pattern (simulating benign atrophy), and foamy gland pattern (which can have bland nuclei).
Urothelial Carcinoma in the Prostate
The malignant neoplasm most likely to enter into the histologic differential diagnosis with prostatic carcinoma is urothelial carcinoma, extending into the prostate from the prostatic urethra or urinary bladder (56,57,58). Primary urothelial carcinoma of the prostate is exceedingly rare, but urothelial carcinoma of the urinary bladder commonly involves the prostate, with a mean incidence of 22% in radical cystoprostatectomies done for urinary bladder urothelial carcinoma. Pathways for secondary spread of bladder carcinoma into the prostate include urethral spread, extravesical extension into prostatic stroma, and by direct growth through the bladder neck into prostatic stroma (58). It is possible to detect urothelial carcinoma involving the prostate in transrectal prostate needle biopsies (56). Microscopically, urothelial carcinoma in the prostate often exhibits prominent in situ spread in prostatic ducts and acini, with solid cylinders, with or without comedo necrosis, being common. Stromal invasion
manifests as irregular nests and cords of pleomorphic tumor cells, which often have a squamoid, eosinophilic cytoplasm. Associated stromal inflammation and sclerosis are frequently pronounced. It is often possible to separate prostatic carcinoma from urothelial carcinoma based on H&E appearances. In difficult cases and in cases of very high-grade carcinoma, an immunohistochemical marker panel to include antibodies directed against PSA, PSAP, p501S, PSMA, NXK3.1, thrombomodulin, p63, and high-molecular-weight cytokeratin can be of substantial value in diagnosing high-grade prostatic versus urothelial carcinoma. Positivity for any of the first five markers favors prostatic adenocarcinoma, whereas the latter three are more typically expressed in urothelial carcinoma (57).
manifests as irregular nests and cords of pleomorphic tumor cells, which often have a squamoid, eosinophilic cytoplasm. Associated stromal inflammation and sclerosis are frequently pronounced. It is often possible to separate prostatic carcinoma from urothelial carcinoma based on H&E appearances. In difficult cases and in cases of very high-grade carcinoma, an immunohistochemical marker panel to include antibodies directed against PSA, PSAP, p501S, PSMA, NXK3.1, thrombomodulin, p63, and high-molecular-weight cytokeratin can be of substantial value in diagnosing high-grade prostatic versus urothelial carcinoma. Positivity for any of the first five markers favors prostatic adenocarcinoma, whereas the latter three are more typically expressed in urothelial carcinoma (57).
Immunohistochemistry in the Diagnosis of Prostatic Adenocarcinoma
The most valuable adjunctive study for the diagnosis of adenocarcinoma of the prostate, particularly minimal adenocarcinoma, is immunohistochemistry with antibodies directed against basal cells (34βE12 and p63) and AMACR (also known as P504S or racemase) (42). Currently, immunostains are employed in 10% to 20% of all prostate needle core cases (42,59).
Basal cell immunostains can be useful as a confirmatory measure in specific circumstances. Basal cell stains are most often ordered in selected cases in which the differential diagnosis is centered on atypia (ASAP), radiation atypia, atypical adenomatous hyperplasia (adenosis), PIN, basal cell hyperplasia, and atrophy. Minimal residual carcinoma after hormonal therapy also can pose diagnostic challenges, and positive immunostains for PSA and pan-cytokeratin, with a negative 34βE12 immunostain, sometimes can be helpful. The p63 immunostain appears to be more sensitive in basal cell detection than the immunostain using the 34βE12 antibody (60). Some have combined p63 and 34βE12 antibodies in a cocktail. While basal cell antibody or antibodies may still be used alone in immunohistochemistry, a trend is for their inclusion in a cocktail with an AMACR antibody.
It would be desirable in diagnosing adenocarcinoma to have a positive marker that is relatively specific for malignant prostatic epithelial cells, in addition to the negative basal cell markers. One such marker, AMACR, is selective and very sensitive for carcinoma, based upon immunohistochemical studies (42,61). About 80% to 100% of prostatic adenocarcinomas stain. Up to 21% of benign prostatic glands also stain, including atrophy, but the staining signal is usually more focal and weaker compared to carcinoma. A misleading result can be obtained with partial atrophy, where many cases have been reported to be AMACR-positive. This finding, combined with focal basal loss and nuclear atypia that may be seen in atrophy, could result in a misdiagnosis of partial atrophy as adenocarcinoma. Most high-grade PIN foci stain, a minority of atypical adenomatous hyperplasia cases stain, and nephrogenic adenoma, which rarely involves the prostate, can also be positive. This AMACR immunostain can be quite helpful, as a confirmatory tool, in cases of minimal carcinoma. It is best interpreted only in histologic context and in conjunction with 34βE12 and/or p63 immunostaining. Cocktails comprised of AMACR and a basal cell marker such as p63 or AMACR/p63/34βE12, with single or two chromogens, are useful for simultaneous assessment of these markers in the same glands (42).
Immunohistochemistry for Predictive Markers for Targeted Therapy
Currently, no therapeutic targets in prostatic carcinoma are identified by immunohistochemical staining. Specifically, immunostains for the androgen receptor, HER2/neu, CD117 (c-kit), and the epidermal growth factor receptor are not routinely employed since they are not markers that are predictive of response to treatment directed against these proteins.
Molecular Genetic Testing in the Tissue Diagnosis of Prostatic Carcinoma
Currently, there are no molecular genetic tests that are utilized in tissue to diagnose adenocarcinoma of the prostate.
Gleason Grading of Adenocarcinoma of the Prostate
The most widely used histologic grading scheme in the United States and worldwide is the Gleason system (37,62). The Gleason grading system is recommended for use by the 2004 World Health Organization classification book and the 2010 AJCC/UICC cancer staging manual (63). It should be applied to all adenocarcinomas primary in the prostate without a history of treatment effect (see below on treatment effect).
Gleason grade of prostatic adenocarcinoma is one of the most important characteristics of prostate cancer in that it provides an indication of aggressiveness of the cancer. Gleason grade is a measure of differentiation of the cancer and is highly correlated with all important pathological and clinical endpoints such as pathological stage, response to therapy, metastasis, and cancer-specific survival. It is a major component of the Partin Tables, D’Amico risk groups, and Kattan nomograms, in prediction of pathologic stage and response to treatment. It is also critical in selection of patients for active surveillance.
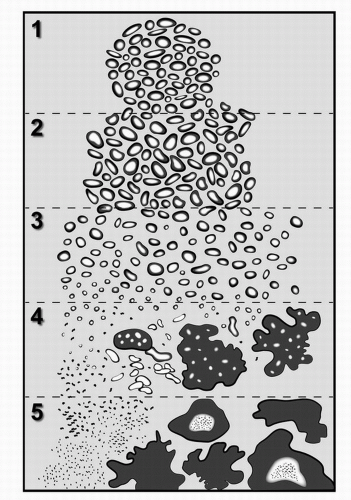
The Gleason grading system is based entirely on the histologic pattern of arrangement of carcinoma cells in H&E-stained prostatic tissue sections. Specifically, the method is one of categorization of histologic patterns at relatively low magnification by the extent of glandular differentiation and the pattern of growth of the tumor in the prostatic stroma. There have been two revisions of the original drawing to reflect new evidence on linkage of Gleason patterns to outcome (37,64). The most current drawing (from Ref. 64) is shown in Fig. 4A.10.
Gleason pattern 1 is a circumscribed nodule of closely packed but separate, uniform, rounded to oval, medium-sized acini (37) (Fig. 4A.10). Most cases of Gleason grade 1 + 1 = score of 2 probably represent adenosis and so this score is rarely used. Gleason pattern 2 is a fairly circumscribed nodule of more loosely arranged glands that are not as uniform as pattern 1. It is most often seen in the context of Gleason score 4 (Fig. 4A.11) or 5. Gleason pattern 3 is comprised of discrete small glands that infiltrate in and around benign glands (Fig. 4A.5). It is most often diagnosed in Gleason score 6 and 7 adenocarcinomas. High-grade Gleason pattern 4 is characterized by fused microacinar glands, ill-defined glands with poorly formed glandular lumina, and cribriform glands (Figs. 4A.6 and 4A.7). It is most often found in Gleason score 7 to 9 carcinomas.
High-grade Gleason pattern 5 shows little to no glandular differentiation and is composed of solid sheets (Fig. 4A.8), cords, single cells, and/or comedocarcinoma. This pattern is usually detected in Gleason score 9 and 10 carcinomas. The five basic grade patterns are used to generate a histologic score (sometimes called sum), which can range from 2 to 10, by adding the primary grade pattern and the secondary grade pattern. The primary pattern is the one that is predominant in area, by simple visual inspection. The secondary pattern is the second most common pattern. If only one grade is in the tissue sample, that grade is multiplied by 2 to give the score.
The Gleason grading system allows for two separate grade patterns in an individual tissue sample, but the histomorphological appearance of prostatic carcinoma is more
heterogeneous than this. Indeed, in one study (65), an average of 2.7 Gleason grade patterns (range 1-5) were found in carcinomas in whole prostate glands. The number of grades assigned depends on tumor sample size and size of the tumor in the whole gland. So, more than two grades are more often observed in TURP chips (28% of cases) compared to needle biopsies (4%-7% of cases) (66), and tumors >1 to 2 cm3 in size tend to have more than two grades.
heterogeneous than this. Indeed, in one study (65), an average of 2.7 Gleason grade patterns (range 1-5) were found in carcinomas in whole prostate glands. The number of grades assigned depends on tumor sample size and size of the tumor in the whole gland. So, more than two grades are more often observed in TURP chips (28% of cases) compared to needle biopsies (4%-7% of cases) (66), and tumors >1 to 2 cm3 in size tend to have more than two grades.
Tertiary high-grade pattern 4 or 5 is important to recognize and report. For needle biopsy tissue where there are more than two patterns present and the worst grade is neither the predominant nor the secondary grade, the predominant and highest grade should be chosen to arrive at a score (67). This approach has been validated in a large clinical series (68). This approach for needle biopsies should also be used for TURP and open prostatectomy cases. In radical prostatectomy tissues, the approach is a bit different: the presence of tertiary high-grade pattern 5 should be noted, but not incorporated into the score. Data from radical prostatectomy cases indicate that a high-grade Gleason pattern 4 or 5 that is a tertiary component occupying <5% of the tumor influences pathological stage and progression rates (69). How to generate an overall score with the tertiary pattern is not established for radical prostatectomy specimens.
Gleason grading should be performed in all prostatic tissue samples, including needle core biopsy specimens. A Gleason grade should be assigned even to needle biopsy cases with minimal prostatic carcinoma, measuring <1 mm in length. Comparisons of Gleason grade in needle biopsy with Gleason grade in the matched whole prostate gland show a 70% to 75% agreement (36). For needle biopsy cases, there are several sources of grading error, including difficulty in appreciation of an infiltrative growth pattern, tissue sampling error (related to the small amount of tissue sampled by needle biopsy and grade heterogeneity), tissue distortion, pathologist experience, and observer variability. Even with extended and saturation needle biopsy protocols, it is difficult to attain perfect agreement between needle biopsy and radical prostatectomy Gleason score.
Assignment of Gleason grade to larger tissue samples, including TURP chips, and open (simple) and radical prostatectomy specimens is often more straightforward than in needle biopsy cases. A distinctive characteristic of carcinoma grade in TURP chips and open (simple) enucleation prostatectomy specimens is that well-differentiated Gleason score 2 to 4 adenocarcinomas are much more common in these tissue specimens compared to needle biopsies and radical prostatectomy specimens. Cautery artifact can generate difficulty in grading carcinoma in TURP chips. The Gleason grade of incidental stage T1a/b carcinoma tends to be somewhat less than the grade in the whole gland since the TURP procedure samples the transition zone where lower grade carcinomas arise.
The Gleason grading system, like all histological grading methods, possesses an inherent degree of subjectivity. Intraobserver and interobserver variability does exist (70). Pathologist training and experience can influence the degree of interobserver agreement. For pathologists, improvement in Gleason grading of carcinoma can be achieved by participation in educational courses at meetings and by use of tutorial programs including Web site programs.
Gleason grading of prostatic carcinoma outside the prostate and in metastatic deposits has been reported, but the Gleason system was not originally designed for this purpose as it is based upon epithelial (carcinoma)-stromal architectural relationships within the prostate.
In general, the Gleason grading system should be applied only to tumor that shows no evidence of treatment effect. For radiation therapy cases, where there is no evidence or minimal evidence of therapy effect, Gleason grading may be utilized. Hormonal therapy can cause pattern alterations resembling high Gleason grades, such that one should not report histologic grade after hormonal therapy, with the exception of finasteride or dustasteride, which are felt to have minimal if any impact on Gleason grade (71).
Experimental grading approaches include quantitation of amount of high-grade (percentage Gleason pattern 4 or 5) carcinoma. Overall, use of the % 4/5 parameter in needle biopsy is limited by a high false-negative rate.
Additional Important Histomorphological Factors in Needle Biopsy Tissue
After Gleason grade, the most important parameter in needle biopsy tissue is cancer extent (46). The amount of prostatic carcinoma in needle biopsy tissue is related to pathologic stage, primary tumor size, and response to therapy. The amount of cancer is reported as number of positive needle cores out of total number of cores, and percentage of prostate needle core tissue involved by carcinoma, and/or linear mm of cancer in the needle core (46). The amount of needle core cancer is used, along with other factors, in nomograms to predict recurrence after radical prostatectomy and small indolent cancers (see below), and in some active surveillance protocols, as one of several factors used as enrollment criteria.
Perineural invasion by carcinoma in needle biopsy tissue is associated with increased pathologic stage in most studies in univariate analysis, but, in general, perineural invasion lacks independent ability in multivariate analysis to predict radical prostatectomy stage once Gleason grade and amount of tumor in needle biopsy tissue are known. Perineural invasion is not used in the Partin Tables to predict pathologic stage and it is not used in any nomogram to predict response to surgery. There is a stronger linkage of perineural invasion in needle core tissue and response to radiation therapy, compared to surgery, although the data are conflicting as to significance.
Pathologic Staging and Margin Status in Radical Prostatectomy Tissues
Pathologic T staging, or determination of anatomic extent of carcinoma, is performed based on histopathologic examination of radical prostatectomy tissue sections and applies only to adenocarcinomas of the prostate and not to sarcomas and prostatic carcinoma variants that are not adenocarcinomas. Pathologic N staging is based upon histologic examination of regional lymph nodes. Pathologic M staging occurs with histologic diagnosis of prostatic carcinoma in nonregional lymph nodes, bone, or other metastatic sites. The 2010 TNM AJCC/UICC staging classification should be utilized (63). Staging is performed separately from margin assessment except when there is intraprostatic incision with a positive margin (with ink on both benign glands and carcinoma). Here the pathologic stage should be designated as pT2+ (67), indicating that the stage is a least pT2 and could be pT3.
Important histopathologic T findings that warrant reporting include the determination of pT2 organ-confined versus pT3a extraprostatic extension (EPE) by carcinoma, focal versus nonfocal (established) EPE, and pT3b invasion into seminal vesicle wall (64). For organ-confined adenocarcinomas, subdivision of pT2 into pT2a, b, and c is felt to harbor little if any prognostic significance (72) but is still retained in the 2010 TNM system. Multifocality and zone of index tumor may be reported but do not provide independent prognostic information. Perineural invasion by carcinoma in the prostate is very common, being seen in 85% to 90% of radical prostatectomy cases, and does not provide prognostic information (73). Extraprostatic extension is defined by the presence of carcinoma in periprostatic adipose tissue or carcinoma in the same plane as the fat. At the anterior and apical prostate and bladder neck regions there is a paucity of fat and in these locations EPE is diagnosed when carcinoma extends beyond the normal arc of benign prostatic glands. It is difficult to impossible to diagnose EPE in distal apical margin sections, due to the ill-defined outer edge of the prostate here and so EPE should not be diagnosed in those sections. For pT3 carcinomas, the amount of extraprostatic cancer is of prognostic significance and should be reported as focal versus nonfocal, and the location (apical, bladder neck, anterior, lateral, posterolateral, and posterior) and number of sites of EPE should be given. Focal EPE is defined as either only a few cancer glands beyond the confines of the prostate (74) or tumor outside the prostate involving less than one high-power microscopic field in one or two sections (75). Nonfocal EPE is more extensive than this. Note that a change in the 2010 TNM scheme compared to the 2002 version is that microscopic bladder neck invasion is now pT3a, not pT4 (63). pT3b carcinoma is tumor in the muscular seminal vesicle wall; carcinoma in soft tissue surrounding the prostate should not be considered pT3b, but rather pT3a. There are several mechanisms for spread of intraprostatic carcinoma into the seminal vesicle wall (76). The most common pathway is EPE by carcinoma into periprostatic tissue at the base of the prostate, with penetration into the outside of the seminal vesicle wall. The second most common type is direct tracking along the ejaculatory duct complex into the seminal vesicles. The third form is characterized by isolated deposits of cancer in lymphvascular spaces in the seminal vesicle wall. Seminal vesicle invasion should be specified as unilateral or bilateral, but it is not necessary to report amount of tumor in the seminal vesicle or the pathway of spread. Finally, additional parameters that should be reported in radical prostatectomy cases include lymphvascular space invasion by carcinoma, which is an independent prognostic indicator (77), and amount of intraprostatic carcinoma. Intraglandular extent of carcinoma is a significant prognostic factor in most series in univariate analysis, but its independent value is controversial in multivariate analyses (46). Quantitation of amount of cancer in the prostate is recommended, with reporting as percentage of the prostate involved by carcinoma and/or as greatest linear dimension of a dominant nodule (if present) (67). Determination of tumor volume by image analysis is not practical for routine reporting. Percentage of the prostate involved by carcinoma, as determined by simple visual inspection, has been shown in several studies to be significant in multivariate analysis in predicting PSA failure after surgery (78,79,80), even for pathologically organ-confined pT2 carcinoma (79,80), and in predicting overall survival (81).
Surgical margin status of the distal apex, bladder neck, and peripheral margins of the prostate is important in prognosis and consideration of adjuvant therapy. A positive margin is a significant risk factor for biochemical recurrence after surgery (82,83), particularly for men with intermediate- and high-risk cancer (83). It should be noted that a positive margin does not necessarily mean that there is residual carcinoma left in the patient. Location and extent of any margin positivity should be given in the surgical pathology report (67). Extent may be reported as number of positive sites and as linear mm of carcinoma at the margin. While the number and extent of positive surgical margins significantly influence the risk of biochemical failure, this quantitation did not improve a nomogram model of failure after radical prostatectomy (82). Some view a positive apical margin as a lesser risk factor for biochemical recurrence compared to other locations, although this is not a universal finding (82).
Pathologic N staging is based on histopathologic evaluation of lymph nodes submitted separately at the time of radical prostatectomy. A histopathologic diagnosis of metastatic prostatic carcinoma in pelvic lymph nodes can be established at frozen section or on permanent sections. Frozen section examination of pelvic lymph nodes is now less commonly requested since there is a low incidence of positive nodes in this current era. If a decision is made to perform a frozen section of lymph nodes, one should be aware of several caveats. First, gross examination of lymph nodes, including assessment of lymph node size, is of limited value due to low sensitivity and specificity (84). Secondly, there is a low sensitivity of around 60% to 70% for identifying lymph node metastasis at frozen section (84). This is due to detection of small metastatic deposits in
permanent sections only and in additionally submitted tissue that was not frozen. Permanent section histologic diagnosis of pelvic lympadenectomy specimens should be based on microscopic examination of a single section from paraffin blocks of all lymph node tissue, and, if possible, all perinodal soft tissue. This is so for several reasons. First, the number of lymph nodes examined is directly related to detection of metastatic disease (85). Second, in about 10% of cases, the only microscopically visualized metastatic deposits will be in soft tissue (84) or in small, grossly invisible lymph nodes or soft tissue (86). These soft tissue metastatic deposits probably are plugs of carcinoma in lymphvascular spaces. Microscopically, prostatic carcinoma in lymph nodes usually resembles one of the patterns in the primary tumor and is usually moderately or poorly differentiated. While extent of cancer in pelvic lymph nodes has prognostic value, the 2010 AJCC/UICC staging scheme does not incorporate extent in pN classification. Furthermore, no “micrometastatic” nodal prostate cancer size has been established. Data on extranodal extension by carcinoma as a prognostic indicator are mixed. Experimental diagnostic tools for detecting prostate carcinoma in lymph nodes include immunohistochemical stains for PSA, PSAP, and cytokeratin and RT-PCR for PSA and PSMA. These approaches are not in routine use and are not currently validated for patient management. Reporting of pelvic lymph nodes should include clinically designated anatomic site of pelvic lymph nodes, number of lymph nodes examined, and number involved by carcinoma (67). Reporting of the diameter of the largest metastatic deposit in mm is also recommended (67). Reporting of extranodal extension by carcinoma is optional (67).
permanent sections only and in additionally submitted tissue that was not frozen. Permanent section histologic diagnosis of pelvic lympadenectomy specimens should be based on microscopic examination of a single section from paraffin blocks of all lymph node tissue, and, if possible, all perinodal soft tissue. This is so for several reasons. First, the number of lymph nodes examined is directly related to detection of metastatic disease (85). Second, in about 10% of cases, the only microscopically visualized metastatic deposits will be in soft tissue (84) or in small, grossly invisible lymph nodes or soft tissue (86). These soft tissue metastatic deposits probably are plugs of carcinoma in lymphvascular spaces. Microscopically, prostatic carcinoma in lymph nodes usually resembles one of the patterns in the primary tumor and is usually moderately or poorly differentiated. While extent of cancer in pelvic lymph nodes has prognostic value, the 2010 AJCC/UICC staging scheme does not incorporate extent in pN classification. Furthermore, no “micrometastatic” nodal prostate cancer size has been established. Data on extranodal extension by carcinoma as a prognostic indicator are mixed. Experimental diagnostic tools for detecting prostate carcinoma in lymph nodes include immunohistochemical stains for PSA, PSAP, and cytokeratin and RT-PCR for PSA and PSMA. These approaches are not in routine use and are not currently validated for patient management. Reporting of pelvic lymph nodes should include clinically designated anatomic site of pelvic lymph nodes, number of lymph nodes examined, and number involved by carcinoma (67). Reporting of the diameter of the largest metastatic deposit in mm is also recommended (67). Reporting of extranodal extension by carcinoma is optional (67).
Pathologic T and N stage, along with radical prostatectomy Gleason grade and margin status, and preoperative PSA are used in a nomogram to predict recurrence after radical prostatectomy (87).
Pathologic Features of Latent Autopsy Prostate Cancers Versus Clinically Detected Prostate Cancers
Latent adenocarcinoma of the prostate is detected at autopsy in a high percentage of cases, with a mean of 25% (range 9%-46%), depending on extent of gland sampling, age of the patient population, and country (88). These clinically unsuspected prostatic carcinomas diagnosed at postmortem examination are of lower grade, stage, and size than clinically detected carcinomas, although there is some overlap in pathologic features between autopsy-detected and clinically detected carcinomas. Most autopsy-detected prostate cancers are well-differentiated Gleason score 2 to 4 acinar adenocarcinomas and organ-confined, with a median tumor volume of 0.24 mL (range 0.01-10 mL) (89). In autopsy cancers high Gleason grade of score 7 or greater is discovered in 16% of men over 60 (90) and up to 16% of men have extraprostatic extension of carcinoma (89). Thus close to about one fifth of autopsy prostate cancers exhibit higher grade and stage characteristics that could have been clinically significant. Similar histopathologic findings have been reported for incidentally detected prostatic adenocarcinomas detected in radical cystoprostatectomy specimens excised for bladder cancer.
There is a subset of clinically detected carcinomas, particularly T1c carcinomas detected due to serum PSA elevation, that have features similar to latent autopsy and cystoprostatectomy-detected prostatic carcinomas. One pathologic definition for potentially insignificant (also called potentially indolent or harmless) prostatic carcinoma, based on examination of radical prostatectomy tissues, is a small (<0.5 mL) carcinoma that is organ-confined, with no high-grade Gleason pattern 4 or 5 (91). Up to 29% of T1c carcinomas fall into this category (92,93).
Pretreatment Prediction of Potentially Insignificant or Clinically Indolent Prostate Cancer
Efforts have been made to develop clinical and pathologic needle biopsy criteria to predict potentially indolent prostate cancer (92,94,95,96,97). One model uses percentage free PSA >15, Gleason score <7, and <3 cores involved, with no core having more than >50% tumor involvement (94). One nomogram utilizes pretreatment serum PSA level, clinical stage, primary and secondary Gleason grade, ultrasound volume, and mm cancer and noncancer in needle core tissue (92). The utility of nomograms to predict indolent cancer has been validated (97), although the prediction is not perfect and there is need for improvement. These models and nomograms may help in the decision-making process between definitive therapy versus active surveillance.
Histologic Variants and Types of Prostatic Carcinoma
The vast majority of primary carcinomas of the prostate are acinar adenocarcinomas. Unusual histologic variants or subtypes account for about 5% to 10% of primary carcinomas (64).
Ductal adenocarcinoma is the second most common subtype of prostatic adenocarcinoma after the acinar subtype. Previously known as endometrioid adenocarcinoma, in pure form it accounts for about 1% of prostatic cancers and when mixed with acinar adenocarcinoma, roughly 5% of prostatic cancers. Microscopically, these are usually papillary and/or cribriform columnar cell adenocarcinomas that can arise centrally (causing urinary obstruction and hematuria), but can also be found peripherally. Most patients present at a more advanced stage and outcome is worse than acinar adenocarcinoma.
Variants of acinar adenocarcinoma include atrophic, pseudohyperplastic, foamy, colloid (mucinous), signet ring, oncocytic, lymphoepithelioma-like, and sarcomatoid carcinoma (carcinosarcoma). Particularly notable for poor outcomes are signet ring carcinoma of the prostate, which is high-grade Gleason pattern 5, and sarcomatoid carcinoma, which may be a homologous spindle cell malignancy or heterologous, with an osteosarcomatous, chondrosarcomatous, or rhabdomyosarcomatous component. In one half of the men with sarcomatoid carcinoma, there is a history of prostatic adenocarcinoma treated by hormonal and/or radiation therapy.
Rare types of prostatic carcinoma include urothelial carcinoma (arising from central prostatic ducts), squamous and adenosquamous carcinoma, basal cell carcinoma, and neuroendocrine carcinomas, including small cell and large cell neuroendocrine carcinomas. It is important to exclude primary urethral and urinary bladder urothelial carcinoma before diagnosing primary prostatic urothelial carcinoma. Squamous cell and adenosquamous carcinomas of the prostate comprise <1% of all prostatic carcinomas and in about two thirds of cases there is a history of hormonal and/or radiation treatment of acinar adenocarcinoma. Average survival is 2 years. Basal cell carcinoma of the prostate includes malignant basaloid proliferations (basaloid or basal cell carcinomas) and also neoplasms that resemble, to a certain degree, adenoid cystic carcinomas of the salivary glands. Small cell carcinoma of the prostate is quite rare and in one half of cases is admixed with adenocarcinoma. The histological appearance is similar to small cell carcinoma of the lung. In one third of cases, there is a history of prostatic adenocarcinoma, followed by hormonal therapy. A similar history is obtained in most cases of large cell neuroendocrine carcinoma of the prostate. Outcome is very poor for neuroendocrine carcinomas of the prostate.
Unusual Primary and Secondary Prostatic Neoplasms
Mesenchymal neoplasms are rare. The most common benign mesenchymal neoplasm is the leiomyoma, and the most common malignant mesenchymal neoplasms are rhabdomyosarcoma in children and leiomyosarcoma in adults.
Hematolymphoid neoplasms may involve the prostate, including leukemia, lymphoma, Hodgkin disease, and multiple myeloma. Leukemic infiltrates almost always indicate secondary spread, and are usually chronic lymphocytic leukemia. About one third of prostatic lymphomas are felt to be primary. The most common histologic type is diffuse large B cell lymphoma.
Miscellaneous neoplasms rarely encountered in the prostate include paragangliomas, melanocytic neoplasms, and germ cell tumors.
Secondary malignancy in the prostate is overall uncommon, but can be seen in a substantial minority of patients with urothelial carcinoma of the urinary bladder, leukemia, and non-Hodgkin lymphoma.
References
1. McNeal JE. Prostate. In: Mills SE, ed. Histology for pathologists, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:923-942.
2. McNeal JE. Anatomy of the prostate: an historical survey of divergent views. Prostate 1980;1:3-13.
3. Franks LM. Benign nodular hyperplasia of the prostate: a review. Ann R Coll Surg 1954;14:92-106.
4. Fine SW, Al-Ahmadie HA, Gopalan A, et al. Anatomy of the anterior prostate and extraprostatic space: a contemporary surgical pathology analysis. Adv Anat Pathol 2007;14:401-407.
5. Ayala AG, Ro JY, Babaian R, et al. The prostatic capsule: does it exist? Its importance in the staging and treatment of prostatic carcinoma. Am J Surg Pathol 1989;13:21-27.
6. Kothari PS, Scardino PT, Ohori M, et al. Incidence, location, and significance of periprostatic and periseminal vesicle lymph nodes in prostate cancer. Am J Surg Pathol 2001;25:1429-1432.
7. Powell MS, Li R, Dai H, et al. Neuroanatomy of the normal prostate. Prostate 2005;65:52-57.
8. Ali TZ, Epstein JI. Perineural involvement by benign prostatic glands on needle biopsy. Am J Surg Pathol 2005;29:1159-1163.
9. Srodon M, Epstein JI. Central zone (CZ) histology of the prostate: a mimicker of high-grade prostatic intraepithelial neoplasia. Hum Pathol 2002;33:518-523.
10. Srigley JR, Dardick I, Hartwick RW, et al. Basal epithelial cells of human prostate gland are not myoepithelial cells. A comparative immunohistochemical and ultrastructural study with human salivary gland. Am J Pathol 1990;136:957-966.
11. Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. J Clin Invest 2007;117:2044-2050.
12. di Sant’Agnese PA. Neuroendocrine differentiation in prostatic carcinoma. Recent findings and new concepts. Cancer 1995;75:1850-1859.
13. Peeling WB, Griffiths GJ. Imaging of the prostate by ultrasound. J Urol 1984;132:217-224.
14. Nazeer T, Kee KH, Ro JY, et al. Intraprostatic adipose tissue: a study of 427 whole mount radical prostatectomy specimens. Hum Pathol 2009;40:538-541.
15. Renshaw AA. Correlation of gross morphologic features with histologic features in radical prostatectomy specimens. Am J Clin Pathol 1998;110:38-42.
16. Sakr WA, Grignon DJ. Prostate. Practice parameters, pathologic staging, and handling radical prostatectomy specimens. Urol Clin North Am 1999;26:453-463.
17. Al-Ahmadie HA, Tickoo SK, Olgac S, et al. Anterior-predominant prostatic tumors: zone of origin and pathologic outcomes at radical prostatectomy. Am J Surg Pathol 2008;32:229-235.
18. McNeal JE, Redwine EA, Freiha FS, et al. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol 1988;12:897-906.
19. Erbersdobler A, Augustin H, Schlomm T, et al. Prostate cancers in the transition zone: Part 1; pathological aspects. BJU Int 2004;94:1221-1225.
20. Cohen RJ, Shannon BA, Phillips, et al. Central zone carcinoma of the prostate gland: a distinct tumor type with poor prognostic features. J Urol 2008;179:1762-1767.
21. Augustin H, Erbersdobler A, Hammerer PG, et al. Prostate cancers in the transition zone: Part 2; clinical aspects. BJU Int 2004;94:1226-1229.
22. Shannon BA, McNeal JE, Cohen RJ. Transition zone carcinoma of the prostate gland: a common indolent tumor type that occasionally manifests aggressive behaviour. Pathology 2003;35:467-471.
23. Erbersdobler JJ, Huhle S, Palisaar J, et al. Pathological and clinical characteristics of large prostate cancers located in the transition zone. Prostate Cancer Prostatic Dis 2002;5:279-284.
24. Garcia JJ, Al-Ahmadie HA, Gopalan A, et al. Do prostatic transition zone tumors have a distinct morphology? Am J Surg Pathol 2008; 32:1709-1714.
25. Augustin H, Hammerer PG, Blonski J, et al. Zonal location of prostate cancer: significance for disease-free survival after radical prostatectomy? Urology 2003;62:79-85.
26. Chun FK, Briganti A, Jeldres C, et al. Zonal origin of localized prostate cancer does not affect the rate of biochemical recurrence after radical prostatectomy. Eur Urol 2007;51:949-955.
27. Haarer CF, Gopalan A, Tickoo SK, et al. Prostatic transition zone directed needle biopsies uncommonly sample clinically relevant transition zone tumors. J Urol 2009;182:1337-1341.
28. Villers A, McNeal JE, Freiha FS, et al. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer 1992;70:2313-2318.
29. van der Kwast TH, Billis A, Amin MB, et al. International Society of Urological Pathology Consensus Conference on Handling of Prostatectomy Specimens. Working group 2—T2 substaging and prostate cancer volume. Mod Pathol 2011;24:16-25.
30. Arora R, Koch MO, Eble JN, et al. Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer 2004;100:2362-2366.
31. Byar DP, Mostofi FK. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer 1972;30:5-13.
32. Kobayashi M, Ishida H, Shindo T, et al. Molecular analysis of multifocal prostate cancer by comparative genomic hybridization. Prostate 2008;68:1715-1724.
33. Mehra R, Han B, Tomlins SA, et al. Heterogeneity of TMPRSS2 gene rearrangements in multifocal prostate adenocarcinoma: molecular evidence for an independent group of diseases. Cancer Res 2007;67:7991-7995.
34. Algaba F, Epstein JI, Aldape HC, et al. Assessment of prostate carcinoma in core needle biopsy—definition of minimal criteria for the diagnosis of cancer in biopsy material. Cancer 1996;78:376-381.
35. Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 2004;17:292-306.
36. Fine SW, Epstein JI. A contemporary study of correlating prostate needle biopsy and radical prostatectomy Gleason score. J Urol 2008;179:1335-1339.
37. Epstein JI, Allsbrook WC Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 2005;29:1228-1242.
38. Ravery V, Boccon-Gibod LA, Dauge-Geffroy MC, et al. Systematic biopsies accurately predict extracapsular extension of prostate cancer and persistent/recurrent detectable PSA after radical prostatectomy. Urology 1994;44:371-376.
39. Bostwick DG, Dundore PA. Normal anatomy and histology. In: Biopsy pathology of the prostate. London: Chapman and Hall Medical, 1997:12.
40. Gaudin PB, Epstein JI. Adenosis of the prostate. Histologic features in needle biopsy specimens. Am J Surg Pathol 1995;19:737-747.
41. Herawi M, Parwani AV, Irie J, et al. Small glandular proliferations: most common benign mimickers of prostatic adenocarcinoma sent for expert second opinion. Am J Surg Pathol 2005;29:874-880.
42. Hameed O, Humphrey PA. Immunohistochemistry in diagnostic surgical pathology of the prostate. Semin Diagn Pathol 2005;22: 88-104.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree