Ambiguous genitalia
AMBIGUOUS GENITALIA: TALKING WITH THE PARENTS
DEVELOPMENT OF THE REPRODUCTIVE SYSTEM
Human Testicular Differentiation
Development of Internal Genital Structures
Development of External Genital Structures
DISORDERS OF GONADAL DIFFERENTIATION
46,XX DISORDER OF OVARIAN DEVELOPMENT
DISORDERS OF CHOLESTEROL AND STEROID BIOSYNTHESIS
Luteinizing Hormone Choriogonadotropin Receptor Gene
Congenital Lipoid Adrenal Hyperplasia
Side Chain Cleavage Cytochrome P450 Enzyme
Virilizing Congenital Adrenal Hyperplasias
3β-Hydroxysteroid Dehydrogenase Deficiency
Defects in Sex Steroid Biosynthesis
17α-Hydroxylase/17-20 Lyase (CYP17) Deficiency
3α-Hydroxysteroid Dehydrogenase Isozyme Deficiencies
Cytochrome P450 Oxidoreductase Deficiency
17β-hydroxysteroid Dehydrogenase Type 3 Deficiency
ENDOCRINE ENVIRONMENTAL DISRUPTORS
Introduction
Normal gonadal differentiation and sex development depend on the meticulous choreography and synchrony of a network of endocrine, paracrine, and autocrine signaling pathways reflecting the actions and interactions of specific genes, transcription factors, and hormones. Perturbations of this intricate network of gene regulation and gene expression governing fetal gonadal development result in disorders of sex development (DSD). Approximately 1 in 4000 infants is born with a DSD.1
Talking with the parents
The initial treatment goal is to determine if there is an underlying or associated life-threatening condition that requires specific urgent treatment. If the child’s gender remains unclear, information needs to be obtained to assist the parents in determining the most appropriate sex of rearing. Usually, this can be accomplished within a matter of hours or days. In more complex instances, the diagnostic process may take longer. In situations in which it is impossible to identify the specific etiology, the general DSD category (see later) provides a basis for decision making.
Factors to be considered in the medical decision-making process include the extent of external and internal reproductive system development, evidence of gonadal functionality (potential for pubertal hormone secretion and fertility), and hormone responsiveness. In some instances, these factors are more relevant than the karyotype. Genes and gene products mapped to diverse autosomal areas of the genome influence the sex development of the developing fetus and child. When consensus has been reached regarding a diagnostic category, available outcome information for that diagnosis should be reviewed. Knowledge of the specific etiology, including details of the diagnosis, enables planning therapeutic interventions and genetic counseling for future pregnancies. Although the exact details are unclear, the extent, timing, and duration of prenatal androgen exposure likely influences CNS differentiation and affects multiple functions. Hence, healthcare providers need to be cognizant that available outcome data to assist in the decision-making processes are limited. Currently accessible information in published reports is largely based on retrospective studies obtained using diverse methodologies.2
During the early dialogues, examining the infant with the parents to identify the specific physical findings of their infant is often beneficial. This can reduce their apprehension, increase their comfort when viewing their infant’s genitalia, and reinforce their perception that their infant’s needs are similar to those of all infants. Information can be presented in a manner that will minimize anxiety and better equip parents to participate in the decision-making process. To provide the best support for their infant, each parent must reach an individual resolution with a commitment to a positive perspective concerning this situation. Discussion of the many concerns (particularly those related to gender identity, pubertal development, sexual orientation, sexual function, and fertility) may be helpful. Honest discussions will engender positive feelings that enhance positive interactions and enable the parents to promote their child’s self-esteem.
Terminology
Under the auspices of the Pediatric Endocrine Society (North America) and the European Society for Pediatric Endocrinology, an international consensus statement was formulated that recommended a revised classification of the medical terminology used for disorders of sex development to avoid confusing and derogatory terms.1 This descriptive classification attempts to be sensitive to concerns of parents and flexible enough to incorporate novel molecular genetic information. The updated classification system integrates molecular genetic considerations into the nomenclature for “disorders of sexual differentiation (DSD)”1 and provides an approach to the diagnostic evaluation.
Terms such as pseudohermaphrodite, intersex, and gender labeling in the diagnosis should be avoided.3 To accommodate all types of DSD, the classification system is broad and includes some conditions that do not present with obvious abnormalities of genital development (Box 5-1). The primary goal of this classification system is to provide a framework for diagnosis, assessment, and care management based largely on sex chromosome status. Currently, microarray and candidate gene analyses are increasingly available and utilized.The DSD categories include sex chromosome DSDs such as 45,X/46,XY (formerly mixed gonadal dysgenesis); ovotesticular DSD (formerly true hermaphroditism); 46,XY DSDs such as disorders of testicular development, disorders of androgen synthesis and action (replacing and expanding the former category of male pseudohermaphroditism), and XY sex reversal; and 46,XX DSDs such as masculinization of the XX individual (replacing female pseudohermaphrodite) and XX sex reversal. Because of the complexities of chromosomal and gonadal development, some diagnoses can be included in more than one of the three major categories. The number of genes identified to be involved in sex development continues to increase. Nevertheless, despite many recent advances the specific molecular etiology of the genital ambiguity in an individual cannot always be identified.
Sex determination
Sex determination is the binary switch that launches the developmental destiny of the embryonic gonads to become testes or ovaries. Sexual differentiation refers to the process through which male or female phenotype develops. The gonads, internal genital ducts, and external genital structures all develop from bipotential embryologic tissues. Each cell in the developing gonad has the potential to differentiate into either a testicular or ovarian cell. How the transcriptome of the undifferentiated cell realizes its pathway to develop into an ovary or testis provides an opportunity to elucidate cell fate decision making. However, “the fate decisions in individual cells are highly coordinated such that cells of discordant fate are rarely seen.”4 Thus, male and female development depends on the regulated orchestration of the expression and interaction of specific genes and gene products. Sex determination is largely influenced through transcriptional regulation whereas secreted hormone and hormone receptors influence sex differentiation.
Through Alfred Jost’s experiments with fetal rabbits in the 1940s and 1950s, the critical requirements for a testis and testosterone for male sexual differentiation were established.5 Chromosomal composition of the human embryo, XX or XY, determines gonadal sex. The genetic locus primarily responsible for this binary switch, the sex-determining region on the Y (SRY) gene on the Y chromosome, was identified through studies of patients with disorders of sexual differentiation. Studies involving creation of transgenic SRY+ mice confirmed the essential role of SRY and provided further molecular understanding of testicular differentiation.6,7
Development of the reproductive system
Urogenital development
The gonads are derived from intermediate mesoderm. In humans, at 4 to 6 weeks of gestation, the urogenital ridges develop as paired outgrowths of coelomic epithelium (mesothelium). The gonads, adrenal cortex, kidney, and reproductive tract derive from the urogenital ridge (Figure 5-1). Several genes are requisite for the development of the bipotential gonad. The Wilms tumor (WT1) gene codes a zinc transcription factor that is expressed in embryonic mesodermal tissues and appears to influence mesodermal-epithelial interactions.8 GATA4 is expressed in the somatic cells of the urogenital ridge and bipotential gonad before showing sex specific expression. Chromobox homolog 2 (CBX2) appears to play a role in early gonadal development and may promote transactivation of steroidogenic factor-1 (NR5A1), which is encoded by NR5A1/SF1. NR5A1 is expressed in the urogenital ridge and appears to upregulate SRY expression. In addition to transcription factors and specific secreted factors (hormones), physical contact with the mesonephros appears to be important for subsequent gonadal differentiation.9
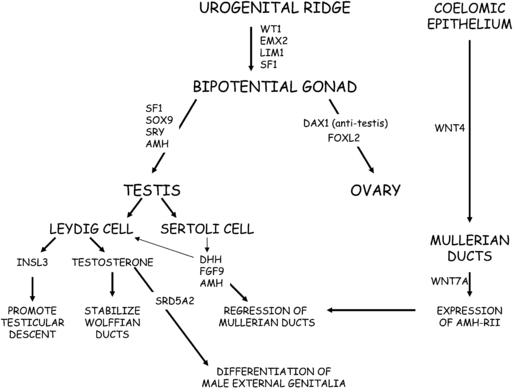
FIGURE 5-1  Cartoon of the genes involved in the process of sexual differentiation. Wilms tumor (WT1), EMX2, LIM1, and steroidogenic factor-1 (SF1) play roles in differentiation of gonad from urogenital ridge. Genes involved in testicular differentiation include SF-1, SOX9, sex-determining region on Y (SRY), and anti-Mullerian hormone (AMH). The dosage-sensitive sex-adrenal hypoplasia congenital critical region on X (DAX1) appears to function as an anti-testis factor. Wnt4 promotes development of the Mullerian ducts, whereas Wnt7a promotes expression of the receptor for AMH (AMH-RII). Sertoli cells secrete AMH, which, acting through its cognate receptor (AMH-RII), promotes regression of the Mullerian ducts. Leydig cells secrete testosterone and insulin-like hormone-3 (INSL3). Testosterone stabilizes the Wolffian ducts and is converted to DHT by 5α-reductase in target tissues to promote differentiation of the prostate and development of male external genitalia. INSL3 is involved in transabdominal testicular descent.
Cartoon of the genes involved in the process of sexual differentiation. Wilms tumor (WT1), EMX2, LIM1, and steroidogenic factor-1 (SF1) play roles in differentiation of gonad from urogenital ridge. Genes involved in testicular differentiation include SF-1, SOX9, sex-determining region on Y (SRY), and anti-Mullerian hormone (AMH). The dosage-sensitive sex-adrenal hypoplasia congenital critical region on X (DAX1) appears to function as an anti-testis factor. Wnt4 promotes development of the Mullerian ducts, whereas Wnt7a promotes expression of the receptor for AMH (AMH-RII). Sertoli cells secrete AMH, which, acting through its cognate receptor (AMH-RII), promotes regression of the Mullerian ducts. Leydig cells secrete testosterone and insulin-like hormone-3 (INSL3). Testosterone stabilizes the Wolffian ducts and is converted to DHT by 5α-reductase in target tissues to promote differentiation of the prostate and development of male external genitalia. INSL3 is involved in transabdominal testicular descent.
Due to their origin as part of the developing urogenital system, ovaries and testes are initially located high in the abdomen near the kidneys. One of the earliest morphologic changes is increased proliferation and size of developing 46,XY gonads. The bipotential gonad consists of at least four cell lineages, which are germ cells, supportive cells, steroidogenic cells, and connective tissue. Although sex determination has been equated with testis differentiation, recent data challenge this dogma. Rather, specific signaling molecules activate or repress gonadal determination for both testes and ovaries. Competition between specific genes and proteins influences cell fate decisions in gonadal development.10 Specific examples include Forkhead transcription factor 2 (FOXL2) vs. SRY-box 9 (SOX9) and SOX9 vs. Wingless-type MMMTV integration site family member (WNT)/β-catenin, which are discussed later.11
Germ cell development
Germ cells are not required for the initial development of ovaries or testes. Rather, the local environment directs the fate of the primordial germ cells. Until approximately 6 weeks of gestation in the human, primordial germ cells proliferate and migrate from the hindgut to colonize the genital ridges. This migration depends on intrinsic motility and external guidance cues as specified by attractive and repulsive signals.12 When this migration process goes awry, the gonadal germ cell population is lacking. Factors important for germ cell migration and colonization of the genital ridges include WNT5A, NANOG, stromal cell–derived factor 1 (SDF1, also known as CXCL12) and its receptor CXCR4.
Meiosis is a sexually dimorphic regulated step governing terminal differentiation of germ cells with meiosis favoring oocytes and active inhibition of meiosis being essential for male germ cells.13 The RNA binding protein Deleted in azoospermia-like (DAZL) plays a role in this branding of germ cells as male or female.14 Factor in germline alpha (FIGLA) and newborn ovary homeobox (NOBOX) are oocyte-specific proteins that appear to repress male specific genes.15 Subsequent stages of germ cell differentiation into a sperm or an egg are closely linked to the cell cycle decision between mitosis and meiosis.16 Meiosis is dependent on retinoic acid, a morphogen, primarily synthesized in the developing gonad.17 Expression of Stimulated by Retinoic Acid (STRA8) increases in the human fetal ovary concomitant with the initiation of meiosis in this tissue, but remains low in the testis. STRA8 is required for pre-meiotic DNA replication and progression through meiosis. In mice, the expression of CYP26B1, a cytochrome P450 enzyme that degrades retinoic acid, prevents meiosis in male germ cells. In human male fetuses, expression of CYP26B1 does not fully account of inhibition of meiosis in the germ cell.18 Expression for NANO2 appears to be restricted to fetal testes.
The Sertoli cells envelope the germ cells to form seminiferous cords at approximately 7 to 9 weeks of gestation in the human XY gonad. The germ cells in the developing testis enter a state of mitotic arrest. In a human XX gonad, germ cell meiosis begins at 10 to 11 weeks of gestation. In the developing ovary, the germ cells initially form clusters connected by intracellular bridges. Selected oogonia enter meiosis and progress through meiotic prophase I (MPI) to arrest at the diplotene stage. The fetal ovary is characterized by the existence of multiple subpopulations of germ cells at different developmental stages. By approximately 20 weeks of gestation, the oogonia clusters break down to form primordial follicles. Primordial follicles destined for future ovulation remain quiescent. By the 24th week of gestation, most oogonia are surrounded by supporting cells. However, apoptosis is the fate for many oogonia. From a peak of 6.8 million oocytes at approximately 5 months of gestation, approximately 2 million are present at birth due to follicular atresia.19 Accelerated follicular atresia contributes to the follicular depletion characteristic of streak gonads in X monosomy.
The internal environment throughout fetal ovary development has the potential to directly influence the fertility of the developing fetus (controlling the size of the ovarian reserve) and the quality of the oocyte that will eventually become her child (by influencing the extent of selection and apoptosis).20 Although not associated with genital ambiguity, mutations in genes governing oocyte and ovarian development cause disorders of sex development characterized by delayed puberty or premature ovarian failure.
During gestation, maternal and paternal alleles are differentially imprinted such that monoallelic expression of specific genes occurs. During this process of imprinting, mature oocytes and sperm are differentially marked reflecting “parent-of-origin” specific methylation patterns. In the primordial immature germ cells, inherited imprints are erased shortly after the germ cells enter the gonadal ridge. Sexually dimorphic methylation imprinting is subsequently reestablished in male and female gametes. This process occurs late in fetal development in the male and postnatally in female germ cells.21,22 The importance of this imprinting process has been elucidated through study of parent-of-origin–dependent gene disorders such as Beckwith-Wiedemann, Prader-Willi, and Angelman syndromes and some forms neonatal diabetes mellitus.
Human testicular development
Testicular differentiation occurs earlier than ovarian development. The testis consists of five cell types: supporting or Sertoli cells, endothelial cells, peritubular myoid cells, steroid-secreting Leydig cells, and germ cells. The first evidence of testicular differentiation is the appearance of primitive Sertoli cells at 6 to 7 weeks gestation in the human fetal testis. Cells, mostly endothelial cells, migrate from the mesonephros and interact with the pre-Sertoli cells to promote development of the testicular cords.23 The testicular cords are precursors of the seminiferous tubules that will contain Sertoli and germ cells. Interactions between endothelial and mesenchymal cells appear to influence development of the testicular cords.24
The binary switch responsible for testicular development is the SRY gene located on the short arm of the Y chromosome. The SRY protein contains a high-mobility group (HMG) domain and is encoded by a single exon gene. Two nuclear localization signals are located in the HMG domain. The SRY protein is expressed in pre-Sertoli cells, where it triggers a molecular switch to induce Sertoli cell differentiation, thus, initiating the process of male sexual differentiation. The HMG domain of the SRY protein binds to the minor DNA groove, where it functions as a transcription factor by bending DNA to presumably permit other proteins access to regulatory regions and to promote assembly of nucleoprotein transcription complexes. A threshold SRY level must be achieved at a critical time during gestation to establish male sexual differentiation. otherwise, the ovarian differentiation pathway is activated.25 Available data suggest that NR5A1 promotes SRY expression.
SRY expression is independent of the presence of germ cells. SRY increases the expression of the SRY-related HMG box-containing-9 (SOX9) gene. Phenotype-genotype studies of humans and mice demonstrate that SOX9 expression is a crucial step, downstream of SRY, in testis development. Upstream from the SOX9 transcription start site, there appears to be a testis specific enhancer element (hTES). Available data suggest that initially phosphorylated NR5A1 and SRY cooperate to activate the hTES leading to increased SOX9 expression; subsequently SRY, NR5A1, and SOX9 maintain SOX9 expression through actions at hTES.26 In addition to SRY, SOX9, and NR5A1, sequential expression of several other genes is required for normal male sexual differentiation. These genes include fibroblast growth factor 9 (FGF9), anti-Mullerian hormone (AMH), dosage-sensitive sex reversal adrenal hypoplasia congenita critical region on X (DAX1),GATA-binding-4 (GATA4), desert hedgehog (DHH), patched (PTCH1), and WNT7A.
Using immunohistochemistry, NR5A1 and SOX9 proteins can be detected in human embryonic gonadal tissue at 6 to 7 weeks of gestation. At this time, SOX9 expression becomes limited to nuclei of Sertoli cells in a 46,XY fetus but remains cytosolic in a 46,XX fetus. NR5A1 and SOX9 protein expression precede AMH expression. After AMH protein expression increases, Wilms tumor (WT1) and GATA-4 protein expression increase in the fetal testis.27 GATA4 belongs to a family of zinc finger transcription factors known as GATA-binding proteins because they bind to a consensus sequence in the promoter and enhancer regions of target genes. Sexually dimorphic expression of Doublesex- and MAB3-Related Transcription Factor 1 (DMRT1) was found in 6- and 7-week-old human fetuses, with expression limited to male fetuses.28
SOX9 induces expression of prostaglandin D synthase (Pgds), an enzyme involved in prostaglandin synthesis.29 In a positive feedback loop, developing Sertoli cells secrete prostaglandin D2, which binds to its cognate receptor to up-regulate SOX9 expression and recruit additional Sertoli cells.30 In addition, SOX9 promotes expression of fibroblast growth factor 9 (FGF9). FGF9 acting through the its receptor, FGFR2, helps to maintain SOX9 expression and promote Sertoli cell differentiation.31 Normal testicular development appears to involve diffusion of FGF9 from the center to the poles of the undifferentiated gonad.32 Through direct and indirect mechanisms, SOX9 interferes with genes promoting ovarian differentiation.
Vascular development in the gonad is sexually dimorphic with the endothelial cells in the developing testis, forming a characteristic pattern consisting of a prominent coelomic vessel on the antimesonephric surface with branches between the testis cords. This blood vessel is absent in the ovary. Peritubular myoid cells are testis-specific smooth muscle–like cells important to structural integrity and development of the testis cords. Factors relevant to peritubular myoid cell differentiation include desert hedgehog (DHH), which is secreted by Sertoli cells and its receptor Patched (Ptch1).33 The peritubular myoid cells surround the Sertoli cells, separating them from the Leydig cells, which are then sequestered in the interstitium. Downstream of SRY, hedgehog signaling plays a role in cell-cell communication and cell fate determination to influence sex dimorphic development in gonads, reproductive tracts, and external genitalia.33
Leydig cell differentiation depends on paracrine signals, including platelet-derived growth factor receptor-alpha (PDGFR-α), DHH, PTCH1, and Aristaless-related homeobox (ARX). NR5A1 is expressed in Leydig cells to promote steroidogenic enzyme genes expression. The number of fetal Leydig cells reflects gonadotropin stimulation because the number is decreased in anencephalic male fetuses and increased in 46,XY fetuses, with elevated gonadotropin concentrations secondary to complete androgen insensitivity.34 Differentiation of adult Leydig cells occurs postnatally.34
By 11 weeks of gestation, the testicular compartments, tubular and interstitial components, and the cell types of interest (Leydig, Sertoli, and germ cells) can be visualized. In human fetal testes, HSD17B3, CYP11A1, and PTCH1 mRNA levels increased significantly through the second trimester without significant changes in CYP17A1, LHR, or INSL3 levels. The most rapid growth in Sertoli cell number appears to occur during the latter half of the first trimester and the second trimester.35
Human ovary development
Although ovarian differentiation has long been considered the default pathway that occurs in the absence of SRY gene expression, accumulating evidence indicates that specific genes influence ovarian differentiation. Genes implicated in ovarian differentiation include wingless-related MMTV integration site 4 (WNT4), forkhead L2 (FOXL2), follistatin (FST), Iroquois-3 (IRX3), bone morphogenic protein-2 (BMP2), and R-spondin-1 (RSPO1).36 FOXL2 functions to repress male-specific genes especially SOX9 beginning in the fetus and continuing through adulthood. FOXL2 and SOX9 expression appear to be mutually exclusive in developing gonads and in gonadal tissue obtained from patients with DSD conditions.37
In the absence of SRY, between 6 to 9 weeks of gestation, R-spondin-1(RSPO1) expression increases in the developing human ovary. RSPO1 is a secreted factor that activates the β-catenin WNT signaling pathway.38 WNT4 expression also increases. RSPO1 and WNT4 appear to stabilize and amplify β-catenin signaling to activate target gene transcription.39 LIN28A, an RNA-binding protein is differentially expressed in the fetal ovary particularly in the germ cells; its expression appears to be restricted to primordial and premeiotic germ cells.40 LIN28 blocks the production of mature let-7, which is a microRNA that interferes with translation of cell cycle regulators and metabolic pathway components.41,42 In the absence of LIN28, let-7 inhibits production of BLIMP1, a factor involved with primordial germ cell development.41 Expression of both let-7 and LIN28A decreases when germ cells transition from the stem cell state to meiosis.41
Two genes, factor in FIGLA and NOBOX, are expressed in fetal or neonatal germ cells where they recruit developing granulosa cells to form primordial and primary follicles. As noted previously, many oocytes degenerate through apoptosis. GATA 4 is expressed in the granulosa cells and is speculated to prevent granulosa cell apoptosis.43 The second trimester human fetal ovary expresses proteins necessary to synthesize and respond to estrogenic, progestogenic, and androgenic signaling.44
Development of internal genital structures
In the male fetus, the Sertoli cells secrete anti- Mullerian hormone (AMH), also known as Mullerian inhibitory hormone (MIH). In human 46,XY fetuses, AMH expression can be detected by 7 weeks of gestation, is not dependent on the presence of germ cells within the testis, and promotes regression of the Mullerian ducts. AMH, a member of the transforming growth factor-β (TGF-β) family, undergoes proteolytic cleavage to become biologically active. AMH binds to its receptor, AMH-RII, on the surface of the Mullerian duct mesenchymal cells to induce increased matrix metalloproteinase 2 expression.45,46 The net result is degeneration and loss of basement membrane integrity of the epithelial and mesenchymal Mullerian cells, leading to regression of the Mullerian ducts.
AMH expression is highly regulated because inappropriate expression in a 46,XX fetus would lead to uterine agenesis. In the 46,XX fetus with absence of both AMH and testosterone, the Mullerian duct derivatives persist and the Wolffian ducts regress. When a female fetus is inappropriately exposed to AMH (as in freemartin cattle), Mullerian duct regression and ovarian masculinization occur. By 12 weeks in the XX fetus, the uterine corpus and cervix have begun to differentiate.47
The prostate, a male accessory sex gland, contributes to seminal fluid plasma and develops from the urogenital sinus. After the initial testosterone-dependent induction of prostate differentiation, subsequent development involves epithelial-mesenchymal interactions that lead to cell differentiation and branching morphogenesis. The requisite signaling molecules FGFs, sonic hedgehog (SHH), BMPs, HOXA13, and HOXD13 are similar to those required for external genital differentiation.48,49
Development of external genital structures
In the 46,XX fetus, in the absence of androgens the urethral folds and labioscrotal swellings do not fuse and develop into the labia minora and labia majora, respectively. The genital tubercle forms the clitoris, and canalization of the vaginal plate creates the lower portion of the vagina. By 11 weeks of gestation, the clitoris is prominent and the lateral boundaries of the urogenital sulcus have separated. Minimal clitoral growth, well- defined labia majora, hypoplastic labia minora, and separate vaginal and urethral perineal openings are present by 20 weeks of gestation.
By 33 days’ postconception, the human fetal adrenal cortex is distinct from the developing gonad. Due to its role as the source of DHEAS for placental estrogen biosynthesis, the fetal adrenal cortex grows rapidly. By 50 to 52 days’ postconception, expression of several steroidogenic enzymes, steroidogenic acute regulatory protein (StAR), 11β-hydroxylase (CYP11B1), 17α-hydroxylase/ 17,20-lyase (CYP17), and 21-hydroxylase (CYP21) in the fetal adrenal cortex have been demonstrated immunohistochemically.50 Recent data indicate that transitory cortisol biosynthesis peaks at 8 to 9 weeks’ gestation.50 This early cortisol biosynthesis coincides with transient adrenal expression of both nerve growth factor IB-like (NGFI-B) and 3β-hydroxysteroid dehydrogenase-2 (HSD3B2).50 At the same time, ACTH can be detected in the anterior pituitary—suggesting the presence of negative feedback inhibition during the first trimester.50 During the time male sexual differentiation begins, this negative feedback inhibition may serve to prevent virilization of female fetuses to ensure normal female sexual differentiation.50,51
Anogenital distance
The anogenital distance (AGD), the distance from the posterior aspect of the scrotum to the anal verge, shows sexually dimorphism. Longitudinal assessment of AGD defined as measurement from the center of the anus to the base of the scrotum in males and to the posterior fourchette in females showed that mean AGD (+/- SD) at birth was 19.8 +/- 6.1 mm in males and 9.1 +/- 2.8 mm in females. In both sexes, AGD increased up to 12 months and maintained the sex dimorphic pattern. AGD also showed positive correlation with penile length at birth and with the increase in AGD from birth to 3 months.52 AGD provides an index of early fetal androgen exposure and masculinization.
Androgens play a time-dependent role in formation, differentiation, and growth of the fetal external genitalia; a role that is especially relevant for male reproductive organ development. Studies among rats suggest that there is a limited time during early fetal development known as the masculinization programming window (MPW). During the MPW the potential for masculinization is determined. Deficiency of androgen or androgen action in the MPW results in reduced penis length, which can not be rescued by postnatal T therapy.53 The MPW predetermines potential organ size, while post-natal androgen action is required to realize normal potential.54
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree