Fig. 21.1
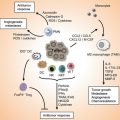
Type I allergic reaction. B B cell, DC dendritic cell, eo eosinophilic granulocyte, IgE immune globulin E, IL interleukin, MC mast cell, Th2 T helper cell type 2, ROS reactive oxygen species. For further explanation, see text
Most of the IgE engage to the high-affinity receptor FcεRI on the surface of mast cells even in absence of Ag. If allergens bind to specific IgE, FcεRI is cross-linked, followed by an inflammatory reaction [15]. Mast cell mediators such as histamine, lipid mediators, and cytokines are released during the effector phase of an allergic reaction and induce typical allergic symptoms. FcεRI is also expressed on basophils which are also able to release allergic mediators being stored in granules [16]. As basophils are able to produce IL-4 as well, they can amplify IgE production [17]. When specific IgE was once built, further exposition to the corresponding allergen elicits an allergic reaction without renewed sensitization [9].
Production of IL-5 by Th2 cells and mast cells activates eosinophils to secrete inflammatory mediators as well as highly toxic proteins and free radicals from their granules [8, 9]. Hours after the early phase of the reaction, the late phase may take place which is characterized by infiltration of further inflammatory leukocytes and eventually a chronic inflammation may be established [18]. The cells involved in allergic reactions reside predominantly in tissues close to the body surface as their actual function is to defend against multicellular parasites which invade primarily into skin and mucosa-associated lymphoid tissue. Therefore, these cells are specialized to evoke Th2 immune responses [8].
21.3 Types of Allergic Diseases
Allergic asthma is a chronic inflammatory disease of the airways caused by inhaled allergens. Symptoms are breathlessness, wheezing and coughing due to bronchial constriction, and increased mucus secretion. It is often accompanied by hyperreactivity of the airways to other stimuli [10, 19]. Allergic rhinitis or hay fever is an allergic inflammation of the nasal mucosa which results in sneezing, itching, and runny or blocked nose and is often combined with allergic conjunctivitis [20]. Atopic dermatitis or eczema is a manifestation of atopy which occurs predominantly among children, showing symptoms like itching, red rashes, and small vesicles on the skin [20, 21]. Food allergies mostly cause diarrhea or vomiting, but they may also affect the respiratory tract and others [8]. Anaphylaxis is a systemic reaction against an allergen with life-threatening symptoms like hypotension or airway constriction [20].
21.4 Molecular Basics of Carcinogenesis
Cancer is a genetic disease in consequence of a number of mutations in somatic cells. Unlimited growth of the mutated cells leads to formation of neoplasms. Tumor cells are capable of invading into tissues and eventually of disseminating and building metastases in distant regions of the body. The clinical phenotype is varying as well as the implications, depending on the type of cancer and the affected patient. Although the incidence of cancer increases with age, tumors occur in every age group [22].
The development of cancer, carcinogenesis, is a multistep process which requires progressive alterations in the genome of normal cells. Mutations can occur spontaneously or can be generated by so-called carcinogens [23]. A carcinogen is an environmental factor like a chemical compound, a biological substance, a virus, or radiation that is able to interact with DNA and cause damages or alterations in the genome. Usually cells have several mechanisms to repair DNA damages. During the process of repair, the cell cycle is stalled, preventing that this mutation is multiplied. If no repair is possible, the cell is destroyed by apoptosis [24]. An abolition of these mechanisms is a precondition for oncogenesis. Therefore, mutations have to occur in genes which are responsible for the control of cell proliferation, differentiation, or apoptosis [25]. Such critical genes can be divided into two groups: oncogenes and tumor suppressor genes [26]. Products of oncogenes are, e.g., transcription factors, growth factors, or their receptors. Tumor cells are characterized by gain-of-function mutations in oncogenes, resulting in overexpression of oncogene proteins and subsequent increased growth [27]. Tumor suppressor genes, or rather their products, have a repressive effect on cell growth. Loss-of-function mutations in tumor suppressor genes result in unimpeded proliferation or evasion of apoptosis [25].
However, one single mutation is not sufficient for the formation of a cancer cell. Carcinogenesis is a multistep process involving several events that incapacitate control of the cell cycle, thereby creating a cell with growth advantages [28]. The initiation process of carcinogenesis, characterized by somatic changes, is followed by the process of promotion. Different promoters like chemical irritants, hormones, or inflammation induce proliferation of the damaged cells and further mutations, as the genome of cancer cells is very unstable [25, 29]. The next step is tumor progression. By means of alteration of cell adhesion molecules and protease activity, cancer cells are capable of leaving the primary tumor and infiltrating into tissues. Subsequently, tumor cells spread through blood or lymphoid vessels and build metastases in distant parts of the body while they are displacing healthy tissue [30].
21.5 Types of Cancers
Pancreatic cancer is one of the cancer types with the poorest prognosis, as mortality rates almost correspond to incidence rates [31]. The most common type is adenocarcinoma which affects the exocrine component of the pancreas, but other components of the pancreas may also be affected. Main causes are smoking, diabetes mellitus, and chronic pancreatitis [22]. Lung cancer is the third leading type of cancer among men and women and the leading cause of death from cancer among men. More than two thirds of the cases are caused by cigarette smoke [31]. Cancers of the colon and rectum represent the second most common type of cancer. Besides the hereditary component, dietary habits are a major risk factor [3, 31]. Skin cancer includes malignant melanoma, basal cell carcinoma, squamous cell carcinoma, and some others [22]. The first one causes more deaths; however, the others are more prevalent, yet with higher curing rates [31]. Meningioma and glioma are the two most common types of brain cancer, whereby the causes are largely unknown [32]. Lymphatic and hematopoietic cancers are, e.g., leukemia, Hodgkin lymphoma, or non-Hodgkin lymphoma. Leukemia is characterized by an abnormal proliferation of leukocytes and can be classified into acute or chronic and myelogenous or lymphocytic forms [22]. Acute lymphocytic leukemia is the most common tumor disease in childhood, whereas the etiology is still not identified [31]. Among reproductive cancer, prostate cancer in men and breast cancer in women are the leading types of cancer. Furthermore, breast cancer is the most frequent cancer-induced cause of death among women. Other common reproductive tumors are tumors of the uterus, cervix, and ovaries [31].
21.6 Antitumor Immunity
In 1970, Burnet and Thomas established the hypothesis of cancer immunosurveillance. It states that, to a certain degree, the immune system is able to detect and destroy tumor cells before they can arise to clinically detectable malignancies. Meanwhile this hypothesis has been expanded to the theory of immunoediting which is comprised of three phases: the elimination phase, the equilibrium phase, and finally the escape phase [33].
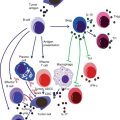
The elimination phase complies with the process of immunosurveillance. Immune cells of innate and adaptive immune response identify tumor cells by so-called tumor Ags [34]. If these are presented to an activated CD8+ T cell, the tumor cell is directly destroyed by the release of cytotoxic proteins. Moreover, antigen-specific B cells produce specific antibodies which can opsonize tumor cells and lead to either antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) [35].
Besides this adaptive immune reaction, there are cells of the innate immune system involved in immunosurveillance which execute antigen-independent immune responses. Among them are natural killer (NK) cells and NK T cells which are able to recognize and directly kill tumor cells [25]. In addition, these two cell types produce IFN-γ which is probably the most important cytokine in antitumor immunity [33]. It acts indirectly by modulating the immune response, e.g., by activation of macrophages or augmentation of T cell response and NK cell activity, and it is able to increase immunogenicity of tumor cells. Moreover IFN-γ itself has anti-proliferative, apoptotic, and angiostatic capacities which directly affect tumor cells [36, 37]. However, cancer cells are capable of defending against these immune mechanisms. Either they lack certain MHC peptides, making them unrecognizable to T cells, or they do not express costimulating signals which lead to T cell tolerance [38]. Hence, if the immune system is not able to kill the entire tumor cells, the process of immunoediting reaches the equilibrium phase, characterized by dynamic dying and generation of further mutated cancer cells [34]. Following Darwin’s rules, those cells, which show surviving advantages through reduced immunogenicity, resist the immune attacks. Thus tumor cells also get shaped and sculpted by immune cells, leading to cell populations that are capable of evading any immune reactions [33]. In this case, surviving tumor cells enter the escape phase. Besides the absent immunogenicity, tumor cells are also able to suppress immune reactivity so that they can proliferate continuously and eventually develop a malignant tumor [38].
Altogether the immunosurveillance hypothesis describes that the immune system is in fact able to fight tumor cells, but also promotes carcinogenesis by sculpting poorly immunogenic mutants.
21.7 Relationship Between Allergies and Cancers in General
The first studies relating to possible associations between allergies and cancer date back to more than half a century [39, 40]. Anyway until now the results have not been consistent, despite various researches in this regard [41].
Regarding cancer in general, there seems to be a balance between studies reporting positive and negative correlations with different types of allergies. While analyses of the Cancer Prevention Study II indicate a slightly decreased risk for people suffering from hay fever or asthma [42], data from the First National Health and Nutrition Examination Survey (NHANES I) show an up to 50 % increased risk of developing any type of cancer [43]. Together with several other studies [19, 21, 39, 44–55], no conclusion can be drawn which identifies the role of allergies in cancer epidemiology. As the term cancer includes diseases of diverse etiologies and a variety of affected tissues, it is necessary to distinguish between different cancer sites as well as specific types of allergy. In the following, those associations which are supported by the majority of studies are presented.
21.7.1 Cancers Positively Correlated with Allergies
Without exception, all of the evaluated studies suggest a positive association between a history of asthma and lung cancer. Without controlling for smoking, a study of 78,000 asthmatic patients found an increased risk for women as well as for men [49]. Another study observed a positive association with asthma, yet no associations with hay fever only, both asthma and hay fever, and any of these conditions [42]. A further survey calculated a lower, but still elevated, risk for asthma when controlling for smoking. An additional analysis of the effect of respiratory drugs taken for the treatment of asthma showed no connection to cancer development [19]. In a Taiwanese study, asthma was the only type of allergy associated with risk of lung cancer [48].
The prevalence of skin cancer was predominantly examined among subjects suffering from atopic dermatitis, for other types of allergy there is only little evidence available. Atopic dermatitis was associated with a clearly increased risk of keratinocyte carcinoma which made up half of all observed excess cancers in this study. Among 6,275 hospitalized patients with atopic dermatitis, not a single case of malignant melanoma was found [50]. Another study involving patients with atopic dermatitis found an increased risk of melanoma as well as of nonmelanoma skin cancer [51].
21.7.2 Tumor-Promoting Effects of Allergies
The positive association between specific types of cancer and allergies is mainly explained by exemplary description of the relationship between asthma and lung cancer. Increased susceptibility to inhaled carcinogens due to impaired mucociliary clearance and pulmonary obstruction and, above all, inflammatory processes are regarded to be responsible for the increased prevalence of lung cancer among asthmatic patients [49, 56–58]. As described before, allergic reactions go along with chronic or subchronic inflammation. There is evidence that tumors predominantly arise at the sites of inflammation and that inflammatory cells and mediators are found in all tumors [59].
Inflammatory reactions are usually triggered by infections. Macrophages, which have detected infectious agents, release chemokines that attract other inflammatory leukocytes, such as neutrophils and further macrophages. Additionally they release cytokines which increase vascular permeability to facilitate migration of attracted cells into afflicted tissues. Leukocyte recruitment is mediated by adhesion molecules and extracellular proteases which relieve movement into the tissue [29]. Since inflammatory responses are supposed to remove the causes as well as to rebuild damaged tissues, an environment rich in growth promoting, but also rich in damage causing, factors is required. Consequently, the conditions for carcinogenesis are established.
Reactive oxygen species (ROS) released by macrophages are capable of causing DNA damages, thus promoting tumor initiation. Permanent cell regeneration raises the probability of carcinogenic mutations [29]. Cancer promotion is supported by growth factors like TGF, IL-1, IL-6, or IL-8. Furthermore, several inflammatory mediators have angiogenic properties or stimulate the production of angiogenic factors. For dissemination, cancer cells exploit the mechanisms that leukocytes utilize for extravasation into inflamed tissues. These are activation of selectin molecules, interactions between integrins and adhesion molecules of the immunoglobulin superfamily, and secretion of proteinases [29].
Apparently, an inflammatory microenvironment is essential for tumor progression, but vice versa, tumors themselves also secrete inflammatory mediators which recruit leukocytes and mediate inflammation [38, 60]. Accordingly, Dvorak described tumors as “wounds that do not heal” [61], indicating that pathogen-induced inflammation is usually self-limiting, while cancer-related inflammation is triggered permanently [29]. Oncogenic mutations that initiate carcinogenesis may also lead to the establishment of an inflammatory environment. The activation of the Ras oncogene by mutation, for instance, leads to the expression of proteins that induce the production of inflammatory mediators [38, 59]. The main mediator cells of tumor-induced inflammation are tumor-associated macrophages (TAM). They are able to release almost all of the cytokines and chemokines required for tumor progression, and their abundance has been shown to correlate with a poor prognosis [29, 62].
One of the key molecules in the connection between inflammation and carcinogenesis is the transcription factor nuclear factor (TNF)-κB. TNF-κB is an endogenous tumor promoter as it is activated immoderately by carcinogenic mutations. In addition, it is a coordinator of inflammation by regulating expression of several proinflammatory and survival factors [59, 62].
21.7.3 Cancers Negatively Correlated with Allergies
The association between a history of allergy and pancreatic cancer seems to be quite definite. Five surveys could demonstrate an inverse association. Holly et al. reported a decreased prevalence of any self-reported allergy among pancreatic cancer patients. This correlation was available for multiple allergens like house dust, plants, molds, animals, and food. Furthermore, with increasing numbers of allergies and increasing severity of symptoms, the risk of cancer development decreased. It should be noted that even after receiving a hyposensitization therapy, allergic patients still showed a reduced risk [63]. Hay fever was correlated with a reduced risk of pancreatic cancer in Turner’s prospective study [42]. Eppel et al. found a risk of pancreatic cancer in allergic patients that was scaled down by more than 50 %, but not for asthma patients. For males separately, the risk was even lower [64]. Another study that additionally investigated a possible association between variants in IL-4 and IL-4 receptor α genes and cancer prevalence found a negative correlation for any allergy, hay fever, and reaction to animals. But variants in the abovementioned genes were not correlated to cancer [65]. A more recent study detected a significantly increased survival of non-resected pancreatic cancer patients with self-reported allergies. In the cohort that has undergone a resection, results were nonsignificant [66].
Cancers of the colon and rectum are less prevalent among individuals that show a history of allergy. Several studies identified allergies to be inversely associated with colorectal cancer. The probability of developing colorectal cancer with any self-reported allergy in an Italian study was lowered, whereas the association was stronger when allergy was diagnosed at age 35 or older. Regarding colon and rectum cancer separately, the risk of rectum cancer development was lower than colon cancer, whereas the latter was not statistically significant [67]. Another case-control study obtained a protective effect of any allergy on cancer development. Self-reported allergy was inversely associated with both colon and rectum cancer [68]. The risk of colorectal cancer calculated by Turner et al. was reduced by more than 20 % among patients suffering from both asthma and hay fever, and less reduction was observed among patients suffering from hay fever only [42]. A prospective study from Iowa involving only women noted an inverse correlation for allergy in general which was the strongest in patients with skin allergies. Moreover, the risk was decreasing with an increasing number of allergies [69]. Allergic rhinitis was negatively associated with rectum cancer among Taiwanese patients, and the association was stronger for males than for females [48]. Combining the cohorts from the Cancer Prevention Study (CPS) I and II, Jacobs et al. calculated a relative risk of 0.83 for colorectal cancer mortality when having both asthma and hay fever [70].
Most studies agree about a decreased risk of tumors of the brain, specifically glioma, being associated with atopic diseases. In a hospital-based case-control study, the prevalence of glioma was reduced in combination with physician-diagnosed history of any allergy and asthma as well as with self-reported allergy to chemicals. Meningioma risk was not associated with any type of allergy. In addition, the risk of acoustic neuroma was positively associated with hay fever, allergy to food, and allergy to other substances [71]. One further case-control study found hospitalized glioma cases to be less likely to suffer from asthma, as well as hay fever, atopic dermatitis, or allergy in general. Moreover, there was a stronger risk reduction in conjunction with use of any allergic medication like nasal spray or antihistamines [72]. Wigertz et al. contrasted the prevalence of allergy among glioma and meningioma cases with noncancerous individuals. They showed a decreased risk of glioma among subjects with asthma, atopic dermatitis, and hay fever. Treatment of hay fever with nasal spray or eye drops was associated with lower risks than non-treated disease. Meningioma risk was only reduced among atopic dermatitis patients [73]. In children having asthma, a 45 % risk reduction could be observed [74]. One case-control study used IgE levels for the measurement of allergy besides a self-reported history of allergy. As IgE levels did not significantly confirm self-reported allergies, odds ratios for the risk of glioma development varied but both implicated a decreased risk [75]. A few years later the same research group reported similar risks for meningioma development [32]. A more recent study confirmed this with an odds ratio of 0.46 for allergen-specific IgE levels and glioma [76]. Besides glioma and meningioma, data from the INTERPHONE study also indicate allergies to protect from acoustic neuroma [77].
21.8 Tumor-Protecting Effects of Allergies
The majority of the presented studies attribute negative associations between allergies and cancers to an enhanced immunosurveillance among allergic patients due to a hypersensitive and hyperactive immune system. This implies that immune cells of allergic subjects are more effective in detecting and destroying cancer cells [48, 53]. The pivotal cells of immunosurveillance are NK cells by virtue of their capacity to carry out ADCC and to produce IFN-γ [37]. There is evidence for increased numbers and activity of NK cells in subjects suffering from asthma or allergic rhinitis [78–80]. Additionally, it could be proved that there is a negative correlation between cancer incidence and natural cytotoxicity which would further explain an improved potential for immunosurveillance among allergic individuals [81].
Besides the classical cells of immunosurveillance, other immune cells may be antitumor effectors as well. Below, critical cells and mediators of allergic reactions and their possible antitumor activities are given. While in nonallergic individuals their activity may be negligible due to low occurrences, their actions may be increased among allergic subjects, explaining a negative correlation between allergies and cancer incidence.
Allergic disorders are marked by increased levels of eosinophils, a condition named eosinophilia, as eosinophils are important effector cells in allergic reactions [82]. A role for eosinophils in immunosurveillance of tumors was considered since they were observed in different tumor infiltrates. Indeed, higher numbers of tissue or blood eosinophils correlated with better prognosis, e.g., improved survival rates in lung and colon cancer [83, 84]. Although eosinophils might contribute to tumor growth by release of VEGF, thereby initiating angiogenesis, in vitro and in vivo studies substantiated rather antitumor activities [6, 85].
Eosinophils are recruited by secretion of IL-5 from Th2 cells and eotaxin-1, a specific chemokine. Particularly IL-5 induces differentiation from CD34+ precursor cells, stimulates synthesis of granule proteins, and activates eosinophil effector functions [86, 87]. These effector functions are mainly mediated by the release of their granule proteins which are highly toxic toward pathogens, as well as toward tumor cells. In vitro studies could prove the direct cytotoxicity of eosinophil cationic protein (ECP) [83, 84, 87]. ECP causes lysis of tumor cells by creating pores in the cell membrane [88]. Further granule proteins like major basic protein or eosinophil peroxidase have indirect antitumor properties in terms of triggering the release of histamine from mast cells. Besides the IL-5 dependent activation, eosinophils are also responsive to specific IgE. As they express IgE receptors on their surface, binding of IgE leads to tumor-specific antibody-dependent cellular phagocytosis (ADCP) [6].
A study which involved lung cancer patients investigated antitumor activities of eosinophils in vitro. For this purpose, eosinophilia was induced by IL-2 treatment in cancer patients. Eosinophils were then purified from blood samples and added to tumor cells. ADCC and direct lysis by eosinophils from IL-2 treated patients were highly increased compared to those of non-treated patients or healthy donors, which did not harm tumor cells at all [83]. This suggests that in fact there are differences in cytotoxic potentials between allergic and nonallergic individuals. The influence of IL-2 was to ascribe to secondary cytokine production because IL-2 has no direct effect on eosinophils, but stimulates lymphocytes. Thus, eosinophil activation was most likely mediated by IL-5.
Another study confirmed the involvement of eosinophils in antitumor immunity in methylcholanthrene-induced fibrosarcoma models. Among IL-5 transgenic mice, which show increased levels of eosinophils, tumor growth and incidence were reduced, whereas among eotaxin-deficient mice, incidence was increased. An even greater increase of incidence was observed in eosinophil-deficient mice. This provides evidence that, at least, chemically induced cancers may be effectively fought and inhibited in growth by eosinophils [86].
IgE is the key mediator of allergic reactions. Binding of IgE to the high-affinity receptor FcεRI on the surface of mast cells and basophils leads to ADCC, whereas binding to the low-affinity receptor CD23 on the surface of macrophages or eosinophils leads to ADCP [6]. Usually IgE is predominantly present in tissues bound to its receptors, but in allergic patients, serum IgE levels are up to ten fold higher than normal [89]. In addition to defense against helminths and hypersensitivity toward allergens, IgE antibodies may also be directed against tumor Ags, thereby mediating antitumor activities. In vitro studies could demonstrate IgE-mediated effector activities against human ovarian carcinoma cells [89, 90]. Furthermore, treatment of mice with IgE targeted on tumor cells resulted in decreased growth of induced cancer. The effect was significantly stronger for IgE than for treatment with IgG. Besides the curative potential of IgE, a protective long-term immunity against the specific tumor cells were observed as well [91]. The incidence of survival was monitored within a case-control study among glioma patients. Those who had elevated levels of IgE were observed to survive on average 9 months longer compared to patients with moderate or borderline IgE levels. Additionally, elevated IgE levels were more common among control subjects than in patients which might support the assumption of an antitumor capacity of IgE [92]. Among pancreatic cancer patients, IgE levels were detected to be five fold higher than in control groups, whereas levels of other Igs were similar. Tumor-specific IgE was found to mediate ADCC against tumor cells, whereas IgE isolated from healthy controls did not [93]. Recapitulating, IgE is an effective mediator of antitumor cytotoxicity as well as phagocytosis of tumor cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree