Abstract
Endocrine therapy is a key component of the adjuvant treatment of early-stage breast cancer. In this chapter, we review the current approach to adjuvant endocrine therapy for early-stage invasive breast cancer and ductal carcinoma in situ. We also review key trials leading to the development of current treatments and review toxicities and unanswered questions. Emphasis is placed on differences in the approaches to adjuvant endocrine therapy in pre- and postmenopausal women.
Keywords
breast cancer, adjuvant endocrine therapy, tamoxifen, aromatase inhibitor, ovarian suppression
The use of endocrine therapy for breast cancer dates back to the early 1900s when favorable results were reported for the management of inoperable advanced breast cancer with surgical techniques for removing sources of estrogen. With the subsequent understanding that breast cancer is a systemic disease in which micrometastases may be present from the time of diagnosis, evaluation of endocrine therapy moved into the adjuvant setting. Studies of oophorectomy after mastectomy for early breast cancer were first reported in the 1950s–1960s. Since then, oral endocrine therapies and nonsurgical methods of ovarian ablation (OA) have been developed and a series of trials evaluating these agents have resulted in adjuvant endocrine therapy becoming a key component of the treatment of early-stage breast cancer. In this chapter, we review the current approach to adjuvant endocrine therapy for early-stage invasive breast cancer and ductal carcinoma in situ (DCIS). We also review key trials leading to the development of current treatments and review toxicities and unanswered questions.
Rationale for Adjuvant Endocrine Therapy
Adjuvant endocrine therapy for early-stage invasive breast cancer is given with curative intent. Meta-analyses performed by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) evaluating individual patient-level data from multiple clinical trials since 1985 have consistently demonstrated that adjuvant endocrine therapy reduces the risk of locoregional recurrence, distant recurrence, contralateral new primary breast cancer, and mortality. The benefits of adjuvant endocrine therapy are substantial, with recurrence rates almost halved and mortality rates reduced by approximately one-third. Benefits are long-lasting, with a carryover benefit continuing up to 5 years beyond the conclusion of therapy.
In the setting of DCIS, adjuvant endocrine therapy can be considered with the aim of reducing the risk of an in-breast recurrence and for contralateral chemoprevention. However, endocrine therapy has no effect on the risk of distant metastases or mortality after DCIS.
Who Is a Candidate for Adjuvant Endocrine Therapy?
The observation that endocrine manipulation can be therapeutic for breast cancer predates the discovery of the estrogen receptor (ER) and progesterone receptor (PR) in breast tumors in the 1970s. It has since been established that the benefit from adjuvant endocrine therapy is limited to hormone receptor–positive breast cancer, making hormonal therapy the first “targeted therapy.” Approximately 75% of invasive breast cancers are ER and/or PR positive, although breast cancers in young women are less likely to be hormone receptor positive. Evaluation of ER and PR status by immunohistochemical assay is required for all breast cancers.
ER status (positive or negative) is the single most important factor predictive of benefit from adjuvant endocrine therapy. The strength of ER expression is predictive of the degree of benefit from adjuvant endocrine therapy, with stronger expression associated with greater benefit. Current guidelines use 1% expression on immunohistochemical assay as the cutoff to define hormone receptor positivity; however, guidelines grant providers discretion with regard to use of adjuvant endocrine therapy in patients whose breast cancers are only weakly hormone receptor positive (defined as 1%–10%) while endorsing it for those whose breast cancers have stronger expression. Data also suggest greater benefit from adjuvant endocrine therapy in patients whose tumors express both steroid hormone receptors (ER and PR positive) than only one receptor. Controversy exists regarding the relative importance of PR expression in predicting benefit from adjuvant endocrine therapy. Analyses from the EBCTCG suggest that, given ER status, PR status does not predict benefit from adjuvant endocrine therapy. However, ER-negative/PR-positive breast cancer is rare, and because PR is an estrogen-regulated gene, there has been debate about whether ER-negative/PR-positive breast cancer is a true entity. Given this uncertainty, adjuvant endocrine therapy is recommended for patients whose breast cancers express both ER and PR and for those whose breast cancers express only one steroid hormone receptor.
Evolving data suggest that factors besides hormone receptor status may affect the degree of benefit from adjuvant endocrine therapy. For example, expression of human epidermal growth factor receptor 2 (HER2) is associated with endocrine resistance. In addition, activation of downstream signaling, such as the PI3Kinase/AKT/mTOR pathway, may be associated with endocrine resistance. Recent trials in the metastatic setting combining endocrine therapy with targeted therapies with the intent of overcoming endocrine resistance have yielded favorable results, and efforts to assess these approaches are ongoing in the adjuvant setting.
Adjuvant Endocrine Therapy: Mechanism of Action
The binding of estrogen to the ER results in a series of downstream steps that modulate transcription of genes responsible for cellular function, tumor growth, invasion, angiogenesis, and survival. Endocrine therapy either antagonizes the ER or causes estrogen deprivation. Types of endocrine therapy used in the adjuvant setting include selective ER modulators (SERMs), aromatase inhibitors (AIs), OA, and ovarian function suppression (OFS) ( Table 54.1 ).
| Selective Estrogen Receptor Modulators | Aromatase Inhibitors | Ovarian Ablation | Ovarian Function Suppression |
|---|---|---|---|
| Tamoxifen 20 mg oral daily | Anastrozole 1 mg oral daily | Oophorectomy | Goserelin 3.6 mg subcutaneous every 28 days |
| Letrozole 2.5 mg oral daily | Ovarian irradiation | Triptorelin 3.75 mg intramuscular every 28 days | |
| Exemestane 25 mg oral daily | Leuprolide 3.75 mg intramuscular every 28 days |
Selective Estrogen Receptor Modulators
Tamoxifen, a nonsteroidal oral SERM that competes for estrogen binding sites in target tissues including the breast, is the only SERM approved for the adjuvant treatment of breast cancer. Notably, its effects on target genes and tissues are mixed, with antagonistic effects in the breast but agonistic effects on other tissues such as the uterus and bone.
Aromatase Inhibitors
Conversion of adrenal androgens to estrogen by peripheral tissues is the primary source of estrogen in postmenopausal women. AIs block aromatase, the enzyme that converts adrenal androgens to estrogen. Currently available AIs are the third-generation reversible nonsteroidal inhibitors anastrozole and letrozole and the irreversible steroidal inactivator exemestane. AI monotherapy is not effective in premenopausal women because it does not block production of estrogen in the ovaries.
Ovarian Ablation and Ovarian Function Suppression
The ovaries are the primary site of estrogen production in premenopausal women. Ovarian estrogen production can be blocked by OA or by OFS. OA is permanent and is accomplished by oophorectomy or ovarian irradiation. OFS is typically reversible and is accomplished with gonadotropin-releasing hormone (GnRH) agonists, also called luteinizing hormone releasing hormone (LHRH) agonists. When GnRH agonists are used, monthly injections are preferred because ovarian suppression may be inadequate with less frequent injections.
Approaches to Adjuvant Endocrine Therapy for Invasive Early-Stage Breast Cancer
Beginning in the 1980s, the EBCTCG meta-analyses established 5 years of tamoxifen as a standard adjuvant therapy for early-stage invasive breast cancer. Although 5 years of tamoxifen remains an option, multiple recent clinical trials have evaluated strategies to improve on the outcomes achieved with 5 years of tamoxifen. In general, strategies that have been evaluated include longer durations of therapy, sequential use of tamoxifen and AIs, AI monotherapy, and the use of OA or OFS. With the expanding data, adjuvant endocrine therapy decisions are increasingly complex and differ by menopausal status.
Evaluating Menopausal Status
Assessment of menopausal status is required for selection of adjuvant endocrine therapy. If postmenopausal status cannot be confirmed, women should be managed as premenopausal. Women can be considered postmenopausal if they meet any of the following criteria: (1) prior oophorectomy; (2) age 60 years or older or (3) under age 60 without a hysterectomy and amenorrheic for at least 12 months in the absence of chemotherapy, SERM therapy, or OFS, with follicle stimulating hormone (FSH) and estradiol concentrations in the postmenopausal range; or (4) under age 60 years with a previous hysterectomy in the absence of chemotherapy, SERM therapy, or OFS, with FSH and estradiol concentrations in the postmenopausal range. It is not possible to determine menopausal status in women who have been treated with OFS. Determining menopausal status after chemotherapy is difficult because amenorrhea may not be a reliable indicator of ovarian function. In this scenario, serial documentation of FSH and estradiol concentrations in the postmenopausal range is required.
Options for Postmenopausal Women With Invasive Early-Stage Breast Cancer
Options for postmenopausal women include monotherapy with tamoxifen or AI for 5 years, tamoxifen for 10 years, AI for 10 years, or sequential therapy for 5 to 10 years.
Tamoxifen Monotherapy for 5 Years: The Historical Standard
In their most recent update, with a median follow-up of 13 years, the EBCTCG investigators demonstrated that approximately 5 years of tamoxifen compared with control was associated with a 39% reduction in the 10-year probability of recurrence of ER-positive early-stage breast cancer (rate ratio 0.61, p < .00001). The risk of recurrence was reduced during years 0 to 4 and 5 to 9, but not in years 10 to 14 of follow-up, suggesting a 5-year carry-over effect. Tamoxifen was also associated with a 38% reduction in the risk of contralateral breast cancer compared with control (rate ratio 0.62, p = .00001). In addition, tamoxifen monotherapy for approximately 5 years reduced breast cancer mortality by 30% (rate ratio 0.70, p < .00001). Mortality benefits from tamoxifen monotherapy for approximately 5 years persist through 15 years of follow-up ( Table 54.2 ). Tamoxifen for 5 years was beneficial regardless of receipt of chemotherapy, nodal status, age at diagnosis, or tumor size. Of note, many of the trials included in the meta-analysis did not require hormone receptor positivity for entry. However, the only factor noted to be predictive of benefit from tamoxifen in the EBCTCG meta-analysis was ER status, and adjuvant tamoxifen is only recommended in the setting of hormone receptor–positive early-stage breast cancer.
| Absolute Reduction in Risk | 5 Years | 10 Years | 15 Years |
|---|---|---|---|
| Breast Cancer Recurrence | 12.4% | 14.2% | 13.2% |
| Breast Cancer Mortality | 3.3% | 6.2% | 9.2% |
Data regarding menopausal status are not available in the EBCTCG meta-analysis. However, women of all ages benefited from tamoxifen with slightly higher proportional risk reductions observed in older women. Although tamoxifen monotherapy for 5 years is now rarely used in postmenopausal women, the EBCTCG meta-analysis established a standard therapy to which newer approaches have been compared.
Tamoxifen Monotherapy for 10 Years
The optimal duration of adjuvant tamoxifen therapy is uncertain. The EBCTCG meta-analysis confirmed that 5 years is superior to a shorter duration. However, because up to half of breast cancer recurrences occur after the completion of 5 years of adjuvant tamoxifen, trials have evaluated an extended course of adjuvant tamoxifen ( Table 54.3 ). Initial studies did not demonstrate a benefit for prolonged treatment, but these were limited by small size, short follow-up, and the inclusion of ER-negative participants. More recently, two larger trials with lengthy follow-up have demonstrated superiority of a 10-year course compared with 5 years. The international Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial and its UK counterpart, the Adjuvant Tamoxifen: To Offer More? (aTTom) trial, together aimed to randomize more than 20,000 women who had completed 5 years of adjuvant tamoxifen to an additional 5 years of tamoxifen or to discontinuation of therapy. Both trials allowed enrollment of women regardless of the tumor’s ER status, but efficacy analyses for the ATLAS trial are limited to the ER-positive subset and, in the case of the aTTom trial, it is estimated that 80% of the ER-unknown participants were likely ER positive. The ATLAS trial demonstrated reduced risk of breast cancer recurrence with extended therapy, with cumulative risks of recurrence during years 5 to 14 of 21.4% in women taking extended therapy compared with 25.1% in controls. In addition, extended therapy was associated with reduced breast cancer mortality in the ATLAS trial with cumulative breast cancer mortality risk during years 5 to 14 of 12.2% in women randomized to extended therapy compared with 15% in controls. The greatest effect of the longer course of tamoxifen on breast cancer mortality in the ATLAS trial occurred in the second decade after diagnosis, consistent with the known carryover effect of tamoxifen. Preliminary findings of the aTTom trial are consistent with those of the ATLAS trial, with late reductions in recurrence and breast cancer mortality with extended therapy ( Table 54.4 ).
| Trial | ER Status of Study Participants | Sample Size (N) | Reduction in Recurrence With Extended Tamoxifen (Yes/No) | Survival Benefit With Extended Tamoxifen (Yes/No) |
|---|---|---|---|---|
| Eastern Cooperative Oncology Group | Any ER status | 193 | No difference for entire study population but longer time to relapse in ER-positive subset | No |
| Scottish Adjuvant Tamoxifen Trial | Any ER status | 342 | No | No |
| NSABP B-14 | ER positive | 1172 | Yes: improved disease-free survival with extended tamoxifen | No |
| ATLAS | Any ER status | 12,894 (6846 ER positive) | Yes | Yes |
| aTTom | ER unknown or ER positive | 6953 (2755 ER positive) | Yes | Yes |
| Years Since Initiation of Tamoxifen Therapy | Recurrence Rate Ratio (95% CI) | Breast Cancer Mortality Rate Ratio (95% CI) | ||
|---|---|---|---|---|
| ATLAS a | aTTom | ATLAS a | aTTom | |
| 5–9 | 0.90 (0.70–1.02) | Years 5–6: 0.99 (0.86–1.15) Years 7–9: 0.84 (0.73–0.95) | 0.97 (0.79–1.18) | 1.03 (0.84–1.27) |
| 10+ | 0.75 (0.62–0.90) | 0.75 (0.66–0.86) | 0.71 (0.58–0.88) | 0.77 (0.75–0.97) |
Eighty-nine percent of the ER-positive participants in both arms of the ATLAS trial were postmenopausal, and the hazard ratio for recurrence was similar in pre- and postmenopausal study participants, although it did not achieve statistical significance in the premenopausal subset, likely due to small sample size. Data regarding menopausal status of the participants in the aTTom trial have not been reported. Although tamoxifen monotherapy is rarely used for postmenopausal women in this era, data from these trials indicate that tamoxifen monotherapy for 10 years may be considered in postmenopausal women, especially if AI therapy is contraindicated.
Aromatase Inhibitor Therapy for 5 Years
To date, the strategy of treatment with a 5-year course of adjuvant AI therapy compared with tamoxifen for 5 years has been evaluated in three major trials ( Fig. 54.1 ). The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial investigators compared 5 years of tamoxifen with 5 years of anastrozole in 6241 postmenopausal women. After a median follow-up of 120 months, the ATAC trial met its primary disease-free survival (DFS) end point favoring anastrozole; however, the benefit was limited to the predefined subgroup of women with hormone receptor–positive breast cancer, which comprised 84% of the trial participants. Among these women, there were statistically significant improvements in DFS, time to recurrence, time to distant recurrence, and the risk of contralateral breast cancer. The reduction in the risk of recurrence with anastrozole compared with tamoxifen was greatest in the first 2 years of treatment but was sustained over time, suggesting a carryover effect for anastrozole also. Despite these benefits, there was no difference in overall survival (OS) between the arms of the ATAC trial.

The Breast International Group 1-98 (BIG 1-98) trial investigators evaluated several adjuvant endocrine therapy treatment strategies in 8010 postmenopausal women with hormone receptor–positive early-stage breast cancer. When it initially opened, women were randomly assigned to either 5 years of tamoxifen or 5 years of letrozole therapy. The study was later expanded to include two additional arms to evaluate switching strategies (letrozole for 2 years followed by tamoxifen for 3 years and tamoxifen for 2 years followed by letrozole for 3 years) with the aim of comparing these new arms to letrozole monotherapy. After an initial efficacy report in 2005 demonstrated superiority of letrozole monotherapy over tamoxifen monotherapy, the trial was amended to allow patients randomized to tamoxifen monotherapy to cross over to letrozole, an option which was taken by 25% of such women. After a median follow-up of 8.7 years, improved outcomes for letrozole monotherapy were observed for the primary end point, DFS, and for secondary end points including OS, distant recurrence–free interval and breast cancer–free interval. The benefits of letrozole monotherapy over tamoxifen monotherapy were present in both node-positive and node-negative patients ( Table 54.5 ). Results of the comparisons of the switching approaches to letrozole monotherapy are described subsequently.
| Trial | Recurrence (95% CI, p ) | Survival (95% CI, p ) | Contralateral Breast Cancer (95% CI, p ) |
|---|---|---|---|
| ATAC a , | HR for DFS | HR for OS | HR for contralateral breast cancer |
| 0.86 | 0.95 | 0.62 | |
| (0.78–0.95, .003) | (0.84–1.06, .4) | (0.45–0.85, .003) | |
| Favors anastrozole | No difference between tamoxifen and anastrozole | Favors anastrozole | |
| BIG 1-98 b , | HR for DFS c | HR for OS | Not reported c |
| 0.82 | 0.79 | ||
| (0.74–0.92, .0002) | (0.69–0.90, .0006) | ||
| Favors letrozole | Favors letrozole |
a Results presented are limited to the ER-positive subset of participants.
b Results presented are for the comparison of letrozole monotherapy to tamoxifen monotherapy.
c DFS end point in Breast International Group (BIG) 1-98 included invasive breast cancer relapse, second primary breast, or nonbreast cancer or death without previous cancer event. Separate HR for contralateral breast cancer in BIG 1-98 not reported.
The National Cancer Institute of Canada (NCIC) MA.27 trial investigators compared 5 years of exemestane monotherapy to 5 years of anastrozole monotherapy in 7576 postmenopausal women with hormone receptor–positive early-stage breast cancer with a primary end point of 5-year event-free survival (EFS). The trial was designed after preclinical evidence suggested that exemestane, a steroidal irreversible suicide inactivator of aromatase, may have greater efficacy than the reversible nonsteroidal AIs. However, after a median follow-up of 4.1 years, there was no difference in the 4-year EFS rate, OS, or disease-specific survival.
Sequential Therapy With Tamoxifen and an Aromatase Inhibitor (or Vice Versa) for 5 Years Total
Switching approaches (tamoxifen followed by an AI or vice versa) for 5 years total have been evaluated in multiple clinical trials to date ( Fig. 54.2 ). As described earlier, after initially randomizing women to either 5 years of tamoxifen or 5 years of letrozole, the BIG 1–98 trial expanded to include two additional arms evaluating switching strategies (letrozole for 2 years followed by tamoxifen for 3 years and tamoxifen for 2 years followed by letrozole for 3 years) with the aim of comparing these arms to the letrozole monotherapy arm. After 8.1 years median follow-up, the sequential approaches were not associated with a lower risk of recurrence than letrozole monotherapy, and there were no differences in OS, distant recurrence–free survival, and breast cancer–free interval.
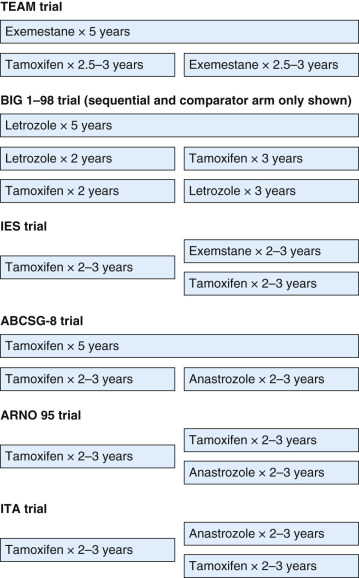
The Intergroup Exemestane Study (IES) investigators randomized 4724 postmenopausal women with early-stage ER-positive or ER-unknown breast cancer who had completed 2 to 3 years of adjuvant tamoxifen to either exemestane or continued tamoxifen to complete a 5-year course of therapy. After a median follow-up of 55.7 months, the study met its primary end point, DFS, with a 24% reduction in the risk of recurrence associated with the switching strategy. On intent-to-treat analysis, there was no statistically significant survival advantage to the switching strategy. However, when 122 participants subsequently confirmed to be ER negative were excluded from the analysis, there was a modest survival advantage for switching to exemestane, with the divergence in risk appearing approximately 2 years after randomization. In addition, contralateral breast cancer was reduced by 43% with the switching strategy.
The Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial was a randomized phase 3 open-label trial initially designed to compare 5 years of tamoxifen monotherapy with 5 years of exemestane monotherapy in postmenopausal women with early-stage breast cancer. However, when data demonstrating superiority of tamoxifen followed by exemestane compared with tamoxifen alone became available from the IES, the TEAM study design was modified to compare 5 years of exemestane to 2.5 to 3 years of tamoxifen followed by exemestane to complete a 5-year course of therapy. Results reported to date include the intent-to-treat analysis of 4868 women who received tamoxifen followed by exemestane and 4898 women who received exemestane monotherapy with a median 5-year follow-up. Similar to the BIG 1–98 trial, there was no significant difference in outcomes for the two arms, and no subgroups benefited from the AI monotherapy approach compared with the sequential approach.
The Italian Tamoxifen Anastrozole (ITA) trial investigators randomized 448 postmenopausal women who had completed 2 to 3 years of adjuvant tamoxifen for early-stage node-positive ER-positive breast cancer to continue tamoxifen or to switch to anastrozole to complete 5 years of therapy. Although the ITA trial closed early after the release of the ATAC trial results, it demonstrated an improvement in relapse-free survival associated with switching to anastrozole. An OS advantage was not demonstrated, although the study was not powered to detect a survival difference.
The Arimidex-Nolvadex (ARNO) trial 95 and the Austrian Breast and Colorectal Cancer Study Group (ABCSG) trial 8 both compared tamoxifen monotherapy for 5 years to tamoxifen for 2 to 3 years followed by anastrozole to complete 5 years of therapy. Each enrolled postmenopausal women with hormone receptor–positive early-stage invasive breast cancer who did not receive chemotherapy. Randomization for the ABCSG 8 trial occurred at baseline, whereas randomization for the ARNO 95 trial occurred within 2 years of initiation of tamoxifen. An initial preplanned combined analysis of these two trials demonstrated a 40% improvement in EFS with the sequential approach, with this improvement largely driven by reduction in distant metastases with anastrozole. After a median follow-up of 30.1 months, intent-to-treat analysis of the ARNO 95 trial revealed improved DFS and OS associated with the switch to anastrozole. In contrast, final results of the ABCSG 8 trial did not reveal significant improvements in recurrence-free survival or OS after 60 months median follow-up. Of note, 18% of participants randomized to tamoxifen monotherapy crossed over to receive anastrozole, which may have masked a benefit of switching. Analysis performed to compensate for selective crossover suggested that switching to anastrozole reduced recurrence by 24% ( Table 54.6 ).
| Trial | Recurrence (95% CI, p ) | Survival (95% CI, p ) |
|---|---|---|
| BIG 1-98: comparison of letrozole × 2 years followed by tamoxifen × 3 years and letrozole × 5 years | HR for DFS 1.06 (0.91–1.23, .48) No difference between arms | HR for OS 0.97 (0.80–1.19, .79) No difference between arms |
| BIG 1-98: comparison of tamoxifen × 2 years followed by letrozole × 3 years and letrozole × 5 years | HR for DFS 1.07 (0.92–1.25, .36) No difference between arms | HR for OS 1.10 (0.90–1.33, .36) No difference between arms |
| IES | HR for DFS 0.76 (0.66–0.88, .0001) Favors tamoxifen followed by exemestane | Intent-to-treat analysis: HR for OS 0.85 (0.71–1.02, .08) After exclusion of 122 participants subsequently confirmed to be ER-negative: HR for OS 0.83 (0.69–1.00, .05) Favors tamoxifen followed by exemestane |
| TEAM | HR for DFS 0.97 (0.88–1.08, .60). No difference between arms | HR for OS 1.00 (0.89–1.14, >.99) No difference between arms |
| ITA | HR for RFS 0.64 (0.52–0.97, .023) Favors tamoxifen followed by anastrozole | HR for OS 0.79 (0.52–1.21, .3) No difference between arms |
| ARNO 95 | HR for DFS 0.66 (0.44–1.00, .049) Favors switching to anastrozole | HR for OS 0.53 (0.28–0.99, .045) Favors switching to anastrozole |
| ABCSG-8 | Intent-to-treat analysis: HR for RFS 0.80 (0.63–1.01, .06) No difference between arms Censored analysis to compensate for selective crossover: HR for RFS 0.76 (0.60–0.97, p not reported) Favors switching to anastrozole | HR for OS 0.87 (0.65–1.16, .34) No difference between arms |
Tamoxifen for 5 Years Followed by an Aromatase Inhibitor for Up to 10 Years Total
Concerns regarding the risk of late recurrence, the uncertain efficacy of prolonged tamoxifen therapy before the ATLAS and aTTom findings, and emerging data demonstrating the benefits of adjuvant AIs led to the evaluation of extended therapy with AIs after 5 years of tamoxifen therapy in three clinical trials ( Fig. 54.3 ). The NCIC MA.17 study was a phase III trial that randomized 5187 postmenopausal women with early-stage hormone receptor-positive breast cancer who had completed approximately 5 years of adjuvant tamoxifen to either placebo or letrozole therapy for 5 years. The study was unblinded after the first interim analysis, and women in the placebo arm were offered the opportunity to cross over to letrozole, an option that approximately two-thirds pursued. Despite extensive crossover, which may dilute findings, this study revealed a 42% improvement in DFS and a 40% improvement in distant DFS with extended adjuvant therapy. There was no effect on OS in the population as a whole, but preplanned subset analysis indicated improved survival in the node-positive subgroup with extended therapy.

Two additional smaller studies have also evaluated the role of extended adjuvant AI therapy in patients who completed 5 years of adjuvant tamoxifen for early-stage breast cancer. The ABCSG 6a study was a continuation of the ABCSG 6 study that compared a 5-year course of tamoxifen to tamoxifen plus aminoglutethimide, an early AI that is no longer in use, demonstrating no advantage to the combination. Postmenopausal women with early-stage hormone receptor–positive breast cancer who completed the ABCSG 6 study were randomized to an additional 3 years of adjuvant anastrozole or to no further adjuvant therapy. After a median follow-up of 62.3 months, intent-to-treat analysis revealed a 38% reduction in the risk of recurrence and a 47% reduction in the risk of distant recurrence with extended anastrozole therapy. Despite these findings, there was no survival advantage associated with extended anastrozole therapy in the ABCG6a study. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-33 trial investigators randomized 1598 postmenopausal women who had completed approximately 5 years of adjuvant tamoxifen for early-stage hormone receptor–positive breast cancer to exemestane or to placebo for 5 years. After the results of the MA.17 trial were reported, the NSABP B-33 trial closed early with approximately half the intended enrollment, and the study was unblinded so that participants in the placebo arm could be offered the opportunity to cross over to exemestane, an option pursued by almost half. Intent-to-treat analysis after 30 months of median follow-up revealed a nonsignificant trend toward improved DFS for the participants originally randomized to exemestane but no difference in survival. These findings are difficult to interpret because they are likely diluted due to extensive crossover and are limited by inadequate power ( Table 54.7 ).
| Trial | Recurrence (95% CI, p ) | Survival (95% CI, p ) |
|---|---|---|
| MA.17 | HR for DFS 0.58 (0.45–0.76, <.001) Favors extended letrozole | HR for OS 0.82 (1.57–1.19, .3) No difference between arms Preplanned subset analysis limited to node-positive participants: HR for OS 0.61 (0.38–0.98, .04) Favors extended letrozole |
| ABCSG-6a | HR for RFS 0.62 (0.40–0.96, .031), Favors extended anastrozole | HR for OS 0.89 (0.59–1.34, .57) No difference between arms |
| NSABP B-33 | HR for DFS 0.68 (95% CI not reported, .07) Trend in favor of exemestane | HR for OS not reported Too few deaths in either arm to draw conclusions No difference between arms |
Aromatase Inhibitor for Longer Than 5 Years
The benefits observed with extended adjuvant therapy in trials such as ATLAS, aTTom, and MA.17, along with the favorable findings for adjuvant regimens including AIs compared with 5 years of tamoxifen monotherapy, raise the question of whether more than 5 years of adjuvant AI therapy is beneficial. Multiple ongoing trials aim to answer this question by evaluating different approaches to extended adjuvant AI therapy. First results of one such trial, MA.17R, were recently published. In this phase III, double-blind, placebo-controlled trial, the investigators randomized 1918 postmenopausal women with a history of hormone receptor–positive early-stage breast cancer who were disease-free after 4.5 to 6 years of adjuvant AI therapy to letrozole or placebo for an additional 5 years. Notably, many participants had also received tamoxifen before AI therapy. The primary end point of the MA.17R trial was DFS, which included local or distant recurrence in addition to new contralateral primary breast cancer. Study findings favored extended letrozole with improved 5-year DFS (95% for letrozole compared with 91% for placebo), although much of this difference was attributable to a reduction in new contralateral primary breast cancer. As expected, bone toxicity was greater in the letrozole arm than placebo; however, participants reported favorable quality of life (QOL) with extended AI therapy. To date, survival data are not mature.
Selecting an Adjuvant Endocrine Therapy Regimen for Postmenopausal Women With Early-Stage Invasive Hormone Receptor–Positive Breast Cancer
Given the numerous trials and approaches to adjuvant endocrine therapy that have been evaluated for postmenopausal women with invasive early-stage hormone receptor–positive breast cancer, selecting the “optimal” regimen can be challenging. Interpretation of the findings and cross-trial comparisons are challenging because of the different patient populations, trial designs, and end points. However, a pattern of reduced risk of recurrence with the use of AI monotherapy or sequential therapy (tamoxifen followed by AI or vice versa) compared with tamoxifen monotherapy is seen in most of the trials. This treatment effect with AI therapy appears quickly, with reduced risk of recurrence noted within approximately 2 to 3 years of initiation of AI therapy. However, data from individual trials regarding the effects of AI therapy on breast cancer mortality are less consistent, with some studies demonstrating a survival benefit and others not. Reasons for this are not certain but may be explained by the fact that many of the studies included predominantly low-risk participants and that tamoxifen therapy itself is quite effective; thus identifying a survival difference would require a very large study and long follow-up.
The EBCTCG investigators recently reported an updated meta-analysis of nine trials evaluating 5 years of adjuvant endocrine therapy with AIs or tamoxifen using individual patient-level data from 31,920 postmenopausal women with ER-positive invasive early-stage breast cancer. Although this analysis does not evaluate extended adjuvant endocrine therapy, it is helpful for the purposes of synthesizing the studies evaluating 5-year approaches and provides some clarity regarding whether adjuvant AI therapy has a survival benefit compared with adjuvant tamoxifen therapy. The key finding from this meta-analysis is that AIs are associated with approximately 30% lower risk of breast cancer recurrence compared with tamoxifen during the period of time when the treatments differ (rate ratio 0.70, 95% confidence interval 0.64–0.77) but not afterward. Furthermore, AI monotherapy for 5 years is associated with a 14% reduction in 10-year breast cancer mortality compared with tamoxifen monotherapy, supporting the notion that AIs, like tamoxifen, have a carryover benefit (rate ratio 0.86, 95% confidence interval 0.81–0.99). In addition, this meta-analysis suggests that the benefit of AI therapy is independent of stage, grade, PR status, and HER2 status and that the third-generation AIs have similar efficacy.
It is important to note that the benefits of AIs compared with tamoxifen are relative risk reductions. Although the proportional benefit of an AI compared with tamoxifen is the same regardless of tumor factors, the absolute benefit differs based on the absolute risk. Understanding the absolute level of risk, the expected benefits of each approach, and the side effect profile can guide clinicians in selecting regimens for individual patients. At this time, there is no best adjuvant endocrine therapy regimen for postmenopausal women, although guidelines suggest inclusion of an AI in the regimen in the absence of contraindications. Criteria for selection of patients in whom to consider extended adjuvant AI therapy have not yet been determined; however, it is reasonable to discuss this option in patients in whom AI therapy is well tolerated, especially in those with a higher risk of recurrence and in whom bone mineral density is favorable.
Options for Premenopausal Women With Invasive Early-Stage Breast Cancer
Recent data have led to rapid changes in the management of premenopausal women with invasive hormone receptor–positive early breast cancer. Accurate assessment of menopausal status is of utmost importance as AI monotherapy is ineffective in women with ovarian function. Current options for premenopausal women include tamoxifen for 5 to 10 years, tamoxifen followed by an AI (if menopause is achieved), and OFS or OA with tamoxifen or an AI.
Tamoxifen Monotherapy for 5 Years
As noted earlier, the EBCTCG meta-analysis revealed a 39% reduction in recurrence and a 30% reduction in mortality with 5 years of tamoxifen. Data regarding menopausal status were not reported, but benefits for tamoxifen were observed for women of all ages including younger than 45 and 45 to 54 years. As is the case for older women, the EBCTCG meta-analysis established 5 years of tamoxifen as a historical standard for premenopausal women.
Tamoxifen Monotherapy for 10 Years
As discussed earlier, the ATLAS and aTTom trials compared 5 and 10 years of tamoxifen, demonstrating reduced recurrence and breast cancer mortality with extended therapy. Only 11% of the ATLAS study participants were premenopausal, and although confidence intervals were wide, subgroup analysis favored extended therapy in the premenopausal group. Data regarding menopausal status of the aTTOm trial participants has not been reported. Despite the inclusion of few premenopausal study participants, these trials are particularly relevant to premenopausal women for whom AI monotherapy is not an option.
Tamoxifen for 5 Years Followed by an Aromatase Inhibitor for 5 Years
Also as described earlier, the MA.17 trial evaluated 5 years of letrozole after 5 years of tamoxifen. Participants were required to be postmenopausal at study entry, but women who were premenopausal at diagnosis and who became postmenopausal after chemotherapy during tamoxifen therapy were eligible. The DFS benefit for extended letrozole therapy was greater in women who were premenopausal at diagnosis (hazard ratio 0.26) than in those who were postmenopausal at diagnosis (hazard ratio 0.67).
Ovarian Ablation or Ovarian Function Suppression Plus Tamoxifen or an Aromatase Inhibitor
For many years, the indication for OA or OFS in adjuvant therapy for premenopausal women with early-stage invasive breast cancer was uncertain. The rationale for considering OA or OFS includes the fact that adjuvant chemotherapy trials have demonstrated improved outcomes in young women who develop chemotherapy-induced amenorrhea (CIA), suggesting that part of the benefit of chemotherapy may be explained by its suppression of ovarian function and that inducing amenorrhea in women who do not experience CIA may be of some benefit. In addition, the collective data described earlier support the superiority of AI therapy over tamoxifen in postmenopausal women with invasive early-stage hormone receptor–positive breast cancer, and this option is only available to premenopausal women in the setting of OA or OFS.
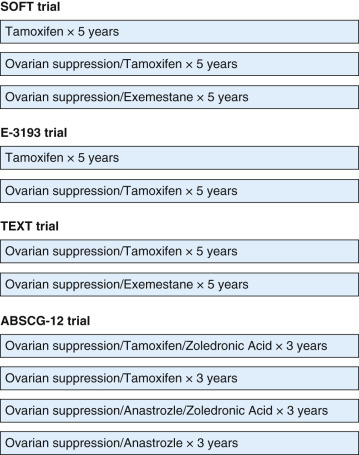
Analysis of OFS or OA in the EBCTCG 2005 update revealed a 4.3% reduction in the 15-year risk of recurrence and a 3.2% reduction in the 15-year risk of breast cancer mortality with the use of OFS or OA. However, the effects of OFS or OA were smaller in trials in which women also received chemotherapy, potentially because chemotherapy could have induced menopause. In addition, hormone receptor status was not documented in all of the trials included in this meta-analysis, potentially confounding the results. The LHRH-Agonists in Early Breast Cancer Overview Group performed a meta-analysis of LHRH agonists in 16 trials including 9022 hormone receptor–positive premenopausal women with early-stage breast cancer. Overall, this meta-analysis demonstrated a greater benefit with OFS than that observed in the EBCTCG meta-analysis with a 12.7% reduction in the risk of recurrence and a 15.1% reduction in the risk of death after recurrence among women who received LHRH agonists in addition to tamoxifen, chemotherapy, or both, but not among those who received LHRH agonists alone. A definite benefit for the addition of LHRH agonist therapy to tamoxifen alone was not confirmed in this meta-analysis because it was not clear how much of the benefit of the combination was attributable to the tamoxifen component, and not all studies included a comparator arm treated with tamoxifen alone. LHRH agonist–based therapy was observed to be approximately equally effective to chemotherapy in this meta-analysis, although the trials included used older chemotherapy regimens. A particular benefit was observed for the use of LHRH agonists after chemotherapy in women younger than age 40 in this meta-analysis, supporting the use of OFS in women less likely to develop CIA. Based on the results of the older studies included in the EBCTCG and LHRH agonists in Early Breast Cancer Overview Group meta-analyses, the role for OFS or OA was unclear, and for many years, it was not routinely recommended as a component of adjuvant endocrine therapy. However, four modern trials have begun to clarify the scenarios in which OFS or OA may improve outcomes ( Fig. 54.4 ).