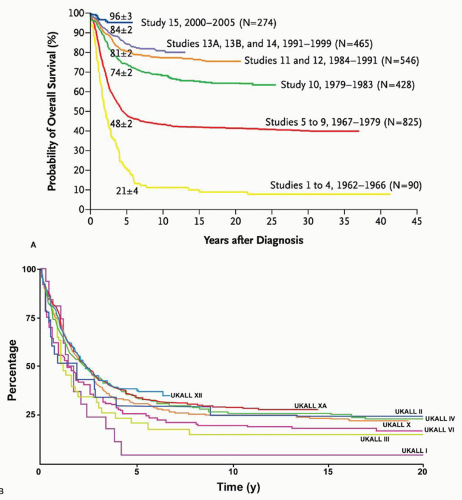
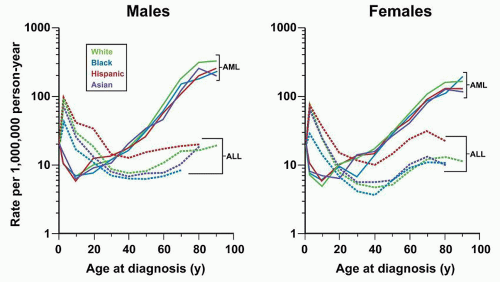
Response to therapy can be assessed by determination of time to attainment of CR, quantitation of early leukemic blast clearance, or detection of MRD. These measurements provide a direct assessment of biologic susceptibility to antileukemic agents, and, as such, prognostic factors based on them have inherently high heuristic power. In addition, prospective evaluation of their utility as predictors of outcome in clinical trials has established that they also have high explanatory power. However, until recently, virtually all of the clinical studies measuring these outcomes have been performed in pediatric ALL. Assessments of response to therapy have become crucial prognostic factors in adult ALL, but remain underutilized in clinical practice. These should aid in refining prognostic models with the goal of improving outcome with ris-kadapted therapy.
Early Complete Remission
Failure to achieve CR within 4 weeks of starting treatment or after one course of induction chemotherapy has been considered an independent unfavorable prognostic factor, confirmed in most adult ALL studies.
7,73 An exception is the international, multicenter UKALL XII/ECOG E2993 trial, which could not confirm its importance in its 1,500 patient cohort.
61 When significant, early CR held predictive value for standard ALL programs as well as for newer, dose-intensified treatment protocols.
5,67,78,106,107,108 Patients requiring greater than 4 weeks to achieve CR were at least twice as likely to relapse, depending on the study. In one study, patients who did not achieve CR by week 4 of induction had a 5-year disease-free survival of 0% as compared with 46% for the remainder of the complete responders.
78 In this study, the number of weeks required to achieve CR was only marginally worse than the Ph chromosome as an unfavorable variable.
Early Leukemic Blast Clearance
Evaluation for persistence of leukemic blasts at even earlier time-points, between days 7 and 21 of induction, has been firmly established as an important prognostic indicator for outcome in pediatric ALL.
109, 110 and 111 Early persistence of leukemic blasts at 7 days after starting induction is thought to represent corticosteroid resistance.
112 In contrast, persistence at time-points after 21 days is considered a reflection of cytotoxic chemotherapy resistance. Numerous prospective studies in pediatric ALL have shown that a substantial unfavorable influence on outcome is associated with the morphologic detection of blood or marrow leukemic blasts persisting during induction therapy at day 7, day 21, or at other time-points in between. Persistence of leukemia was usually defined as the finding of greater than 1 × 10
9/L blast cells in a peripheral blood sample or leukemic blasts greater than 5% of normal cells in a marrow specimen.
Although pediatric protocols already incorporate early treatment response assessment by a day 7 or day 14 bone marrow examination into risk classification, similar data in adult ALL are limited.
113 Results from the few reported studies agree with findings from the pediatric experience. Sebban et al. prospectively evaluated persistence of marrow blasts, defined as greater than 5% of nucleated cells, at day 15 of induction for influence on outcome.
107 Among 437 adult ALL patients, one third were found
to have persistent blast cells. These patients were less likely to achieve CR after 4 weeks of therapy. Even among only the patients who achieved CR within 4 weeks, an otherwise favorable feature, those who had persistent blasts at day 15 fared significantly worse. Among all complete remitters, the 5-year disease-free survival was 34% for those who cleared marrow blasts at day 15, compared with 19% for those who did not. Annino et al. and the Italian collaborative study group evaluated the early corticosteroid response by giving a 7-day course of prednisone immediately before induction.
9 The pre-induction response was previously shown to have strong prognostic value in pediatric ALL.
114,115 In the adult ALL study, prednisone response was defined by reduction of peripheral blood blasts to less than or equal to 1 × 10
9/L. Lack of a prednisone response was found to affect overall survival negatively, as it did in the pediatric ALL studies, and, among CR patients, it also adversely influenced remission duration.
Minimal Residual Disease
MRD refers to the post-remission persistence of leukemia that cannot be detected by histomorphologic assessment. Immunophenotypic, cytogenetic, or molecular techniques can be used to detect MRD.
116 Samples from peripheral blood are generally one log10 less sensitive than bone marrow, perhaps as post-treatment
BCR-ABL transcripts from peripheral blood clear more rapidly,
117 but may have comparable sensitivity in T-lineage ALL.
118,119,120 Fluorescence in situ hybridization is better than conventional banding analysis for detecting chromosomal translocations and numerical abnormalities, but both techniques are limited by low sensitivity.
121, 122, 123 and 124 Flow cytometry, used in four or more color combinations and taking advantage of new reagents, detects aberrant antigen expression on leukemic cells and can unambiguously distinguish one leukemic blast among more than 104 normal cells in 90% of all patients.
125,126 This level of sensitivity is sufficient for prognostically significant MRD detection.
127 Additionally, flow cytometry is rapid, reliable, and allows accurate quantitation, and the technical requirements for the assay are already in place at most centers, making it an attractive diagnostic tool. The most sensitive assays for detecting MRD, however, are based on PCR techniques, which can detect one leukemic blast in up to 106 normal cells. PCR targets using fusion gene transcripts such as
BCR-ABL,
ETV6-RUNX1,
AFF1–
MLL, and
E2A-PBX are relatively standardized, but each alone can only be used for patient subsets.
128, 129 and 130 PCR targets based on immunoglobulin and T-cell receptor gene rearrangements can be used to detect MRD in theoretically all patients, but lose sensitivity if consensus rather than patient-specific primers are used.
128,131 On the other hand, generating patient allele-specific primers requires DNA sequencing capability. Although a number of clinical studies have suggested the prognostic significance of threshold values of MRD,
132,133 early experience with accurate quantitation of leukemia-specific residual disease with the PCR assay was fraught with technical challenges, and standardization of assay sensitivity among testing facilities was of major concern.
130 Where available, however, real-time quantitative PCR technology utilizing newer automated methods has proven to be technically simple, yielding precise and consistent quantitation of residual leukemic clones, and is gaining widespread acceptance.
134,135 Information from pediatric ALL trials using PCR and flow cytometry in tandem may help determine more definitively the role of each for evaluating MRD.
136The prognostic utility of MRD detection was first described in studies conducted on children with ALL.
109,128,137 Three of the pediatric studies stand out for having large patient numbers, inclusion of both B- and T-lineage ALL, and for including patients with poor risk features.
127,132,133 The European studies were multicentered and used PCR detection of patient-specific antigen receptor gene rearrangement, whereas Coustan-Smith et al. used multicolor flow cytometry in an unselected patient cohort treated at the St. Jude Children’s Research Hospital. MRD was determined at the end of induction and at various time-points thereafter. The testing was technically challenging, as 1,254 of 1,914 (66%) combined patients treated during the study period did not have suitable PCR targets, serial PCR performed, or an immunophenotype suitable for MRD assessment. Nonetheless, all three studies demonstrated an unfavorable influence of persistent MRD on relapse-free survival. By multivariate analyses in the St. Jude study, sequential detection of persistent MRD remained a poor prognostic marker after controlling for age, WBC count, and adverse cytogenetics including the Ph chromosome and
MLL gene rearrangement.
127 Detection of MRD during maintenance therapy tended to have the strongest positive predictive value for relapse. Conversely, absence of MRD at the end of induction negated the unfavorable influence of persistent leukemic blasts at day 7 as a prognostic factor.
Studies assessing the predictive value of MRD in adult ALL have generally echoed findings of pediatric studies.
138, 139 and 140 Mortuza et al. reported results for 66 Ph-negative adult B-lineage ALL patients treated at a single institution, for whom MRD was assessed with PCR using consensus immunoglobulin heavy chain gene primers.
138 Detection of residual leukemia at the 10-3 level, the limit of sensitivity of the assay, independently correlated with inferior disease-free survival when measured 3 to 5 months after induction therapy. The kinetics of MRD clearance has also gained much attention with respect to predicting relapse risk. Among the 196 standard-risk ALL patients monitored by quantitative PCR during their first year of treatment in the German Multicenter Acute Lymphoblastic Leukemia (GMALL) study, the 3-year relapse rate was 0% if MRD declined rapidly to lower than 10
-4 at day 11, or below detection threshold at day 24, compared with a relapse rate of 94% if the MRD was at a level of 10
-4 or higher until week 16.
141 Based on these results, they defined three risk groups based on MRD assessment in this otherwise homogeneous standard risk group. Scheuring et al. suggested that the magnitude and rate of
BCR-ABL increase, as well as the absolute number of transcripts, could predict relapse within 2 months.
142 Another large cooperative group study reported that a 2-log reduction of MRD after induction, and a greater than 3-log reduction after consolidation therapy were associated with a 2-year actuarial probability of overall, disease-free, and relapse-free survival of 48%, 27%, and 38%, respectively, compared with 0% survival with smaller reductions.
143 The increasingly recognized prognostic significance of MRD has prompted a large cooperative group study to evaluate prospectively the utility of MRD in selecting patients for stem cell transplantation in first CR as a model for risk-adapted treatment strategy.
144