time between viral infection and the development of the detectable antibody response.
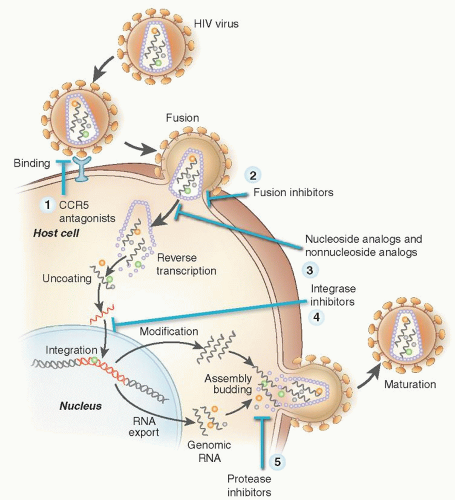
peptide it requires twice-daily subcutaneous dosing and is used uncommonly. Raltegravir (RAL) and elvitegravir (EVG) inhibits the viral-specific enzyme HIV integrase that inserts the viral DNA into the host cell DNA.
analogs, have little or no effect on hematopoiesis.31, 48 It is important to note that HAART itself improves anemia as shown in a single center retrospective series in the HAART era. HAART was associated with hemoglobin levels >140 g/L in 42% of patients, irrespective of use of zidovudine as part of HAART regimen, compared with 31% of patients who did not use HAART.49
TABLE 64.1 HEMATOLOGIC TOXICITIES OF ANTIRETROVIRAL DRUGS | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree