Key points
- •
Percutaneous ablation of bone and soft tissue metastases may be performed for palliation of pain, prevention of complications due to progressive disease, and local disease control in nonsurgical patients.
- •
Patients treated for palliation of painful metastases should have at least moderate pain levels, pain referable to a limited number of metastatic lesions on imaging, and target lesions separated (or separable) from critical normal structures.
- •
Preablation workup should include laboratory assessment and correction of coagulation parameters and platelets, pain and quality of life assessments using standardized scales, and focused physical examination to enable correlation of painful site(s) with imaging findings. Preablation imaging should guide safe percutaneous access to the bone or soft tissue metastasis and assessment of nearby vital structures, particularly nerves, to be avoided or monitored during treatment.
- •
Ethanol ablation offers inexpensive, relatively effective treatment of painful lesions with the disadvantage of unpredictable diffusion of the ablative agent. Radiofrequency ablation offers fast, effective ablation with minimal bleeding risk; however, the procedure cannot be monitored easily, and patients often experience increased periprocedural pain. Cryoablation provides safe, effective ablation with easy monitoring of the ablation zone with CT or MRI, less periprocedural pain, and simultaneous activation of numerous applicators to treat large tumors; its disadvantages include larger applicator diameters, greater expense, and longer procedures. Microwave ablation, laser ablation, and high-intensity focused ultrasound are emerging ablation alternatives.
- •
Cementoplasty provides structural support to ablated metastases at risk for fracture. Although it is frequently used as an adjunct to ablation, it also provides an independent anesthetic effect.
Background
Percutaneous ablation of skeletal and soft tissue metastases has become increasingly common over the past several years. This rise reflects the clinical challenges multidisciplinary teams face in treating patients with metastatic disease. First, a large number of cancer patients have metastatic disease to bone, including up to 85% of patients with the three most common cancer types—lung, breast, and prostate. Skeletal metastases remain an important source of morbidity for these patients, leading to pain, pathologic fracture, and neurovascular compromise. Additionally, bone-related cancer pain is frequently undertreated, with up to 79% of patients experiencing severe pain before adequate palliative efforts are initiated. Patients with skeletal metastases have a poor prognosis in general, with median survival of less than 3 years and 5-year survival varying from 5% to 40% depending on tumor histology. , Treatment options facing oncology patients with musculoskeletal metastases and the multidisciplinary teams that care for them are numerous.
Most patients with diffuse metastatic disease are treated systemically. Chemotherapy, hormonal therapy, and biological agents may be administered, depending on tumor type. These agents are critical to attempt to limit metastatic progression and target micrometastatic disease. Oral analgesics, including opioids and nonsteroidal antiinflammatory drugs, provide sufficient pain relief for many patients with metastases to bone and soft tissue. In those with advanced malignancies, however, high-dose opioids may cause significant nausea, constipation, and sedation. Bisphosphonate medications, which have an osteoclast-targeted effect, are increasingly employed to reduce the risk of skeletal-related events in patients with skeletal metastases. Radiopharmaceuticals also play a role in the treatment of certain skeletal metastases. ,
The standard of care treatment for patients with limited, painful skeletal metastases is external beam radiation therapy. The majority of patients experience a reduction in pain, but approximately 30% fail to experience pain relief, and those that respond to treatment have a median duration of response of 12–28 weeks. Limitations in normal tissue tolerance prevent retreatment of painful metastases within previously irradiated fields.
Surgery for skeletal metastases is usually reserved for cases of impending fracture or after pathologic fracture. Additionally, spinal metastases causing neurologic compromise due to spinal cord compression should be treated with surgical decompression and adjuvant radiotherapy. Less commonly, surgical metastasectomy is performed in patients with limited metastatic disease to bone or soft tissue. , Comorbidities of patients with advanced malignancies make many patients poor surgical candidates. Furthermore, expected postoperative morbidity and recovery times may limit surgical approaches in these patients.
Given the limitations of the conventional therapies above for musculoskeletal metastases and successes of ablation in treating metastases in liver and lung, percutaneous ablative techniques have been applied to the treatment of bone and soft tissue metastases. Thermal device-based techniques, including radiofrequency ablation (RFA), cryoablation, microwave ablation, and laser ablation, have been used. In addition, chemical ablation with ethanol, noninvasive treatment with focused ultrasound, and bone consolidative treatment with cement instillation (cementoplasty and vertebroplasty) have also found a role in the treatment of these patients.
Indications and contraindications
Percutaneous ablation of bone and soft tissue metastases is broadly used to treat four groups of patients ( Table 28-1 ). First, patients with limited painful metastases that are refractory to conventional therapy are well suited to palliative ablative therapy. Similarly, patients who refuse conventional therapy may benefit from ablation. Second, skeletal or soft tissue metastases that place patients at risk for future morbidity, either due to impending pathologic fracture or potential for direct invasion of an adjacent critical structure, are appropriate targets for ablation. Third, percutaneous ablation of limited metastatic disease to bone and soft tissue in patients who are nonsurgical candidates may be performed to obtain local control of all clinically detectable disease. Finally, ablation may be used to treat hormonally active or hemorrhagic symptomatic metastases.
| Indications |
|---|
| Palliation of painful metastases |
| Local control of oligometastatic disease |
| Prevention of complication from local progression |
| Fracture risk |
| Neurologic compromise |
| Palliation of hormonally active or hemorrhagic metastases |
Cementoplasty may be performed alone or in conjunction with tumor ablation. The cytotoxic effect of the cement polymer is limited, and cementoplasty should not be used with curative intent alone. It is indicated to provide pain relief and bone strengthening in painful, osteolytic metastases within the axial skeleton. It may also be used in focal skeletal lesions or pathologic fracture sites due to hematologic malignancies, including lymphoma and multiple myeloma. The cement is resistant to compressive forces, as experienced by weight-bearing bones like vertebrae and acetabula, but of limited benefit for the mixed tension and torsion forces to which diaphyses of long bones are subjected.
Percutaneous ablation of bone and soft tissue metastases has few, nonspecific absolute contraindications ( Table 28-2 ). These include uncorrectable bleeding diathesis, intolerance of the level of anesthesia required for the procedure, and inability to access the targeted tumor safely from a percutaneous approach. Active infection is another contraindication to these sterile procedures. Furthermore, patients who have spinal metastases causing spinal cord compression with resultant neurologic compromise should be treated with surgical decompression and/or radiotherapy.
| Contraindications |
|---|
| Absolute |
| Bleeding diathesis |
| Inability to tolerate anesthesia |
| No safe percutaneous access |
| Active infection |
| Relative |
| Widespread metastatic disease |
| Mildly painful metastases |
| Close proximity to vital structures |
Relative contraindications include diffuse skeletal or soft tissue metastases for which systemic therapy would be more appropriate than a focal approach. Those patients with widespread skeletal metastases may be candidates for palliative ablation, however, if they have a limited number of target lesions that are particularly painful or at risk for causing significant morbidity because of their locations. In addition, musculoskeletal metastases causing only mild pain are usually not appropriate for ablation. Finally, tumors in proximity to critical structures that cannot be displaced or cannot be monitored sufficiently to allow safe tumor ablation may not be appropriate targets. Cementoplasty should be performed cautiously in osteolytic lesions with largely disrupted bony walls along the epidural space or a joint space.
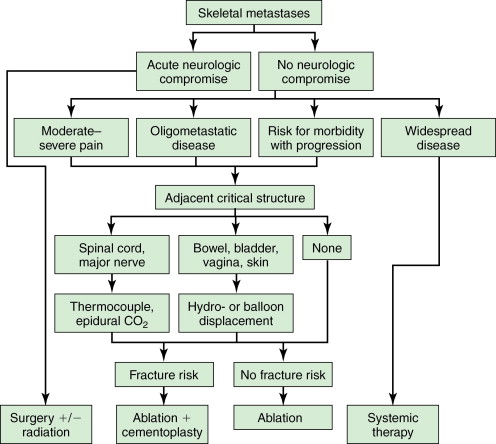
A simple algorithm may be applied to the treatment decisions regarding skeletal metastases in most patients ( Figure 28-1 ).
Patient selection
Successful percutaneous treatment of metastases outside of lung and liver requires proper patient selection ( Table 28-3 ). ( Figure 28-2 ) The preablation workup should include a focused history and physical examination, limited laboratory assessment, and high-quality cross-sectional imaging.
| Selection criteria |
|---|
| Limited metastatic disease (1–3 sites) |
| Moderate pain (≥4 on 10-point scale) |
| Metastasis on imaging corresponding to painful site |
| Target lesion remote (or can be separated) from vital structures |
History
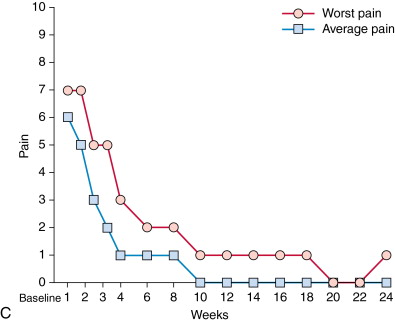
Brief review of a patient’s oncologic history, including previous local and systemic therapies and current disease burden, is critical in the evaluation of each patient considered for local tumor ablation. Prior consultation with an experienced oncologic surgeon avoids inappropriate application of ablation in surgical candidates. In patients referred for control of oligometastatic disease, assessment of a patient’s disease-free interval prior to the development of metastases or rate of metastatic disease progression may offer some prognostic information and help set appropriate expectations for the patient and referring physician. In patients referred for palliation of painful metastatic disease, initial assessment of pain level is crucial. Patient pain levels should be recorded using a standardized visual analog scale, such as the Brief Pain Inventory (BPI) short form. , This includes patients’ self-assessment of their best, worst, and average pain scores. Ablation should be performed on those patients with moderate to severe pain, meaning those with pain levels of 4 or greater on a 10-point scale. Ablation may not be as effective in those with mild pain levels, as they are difficult to improve on and are usually adequately controlled with oral analgesics. Similarly, patient assessment of quality of life parameters should be performed. These assessments should occur both before and serially in the months after ablation to measure the effectiveness of the procedure. Moreover, measurement of a patient’s use of analgesics before and after ablation may demonstrate effectiveness of the procedure. Also, a patient’s activity level may influence the decision of whether to perform adjunctive cementoplasty to consolidate ablated bone. Finally, a patient’s medication list should be reviewed for drugs that increase bleeding risk.
Physical examination
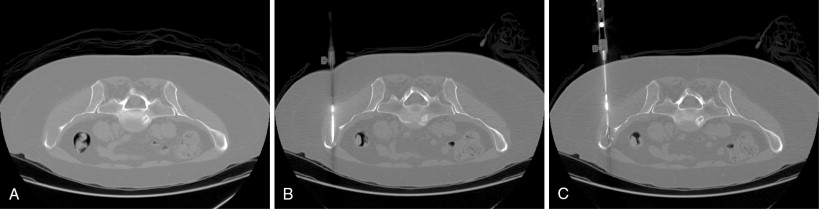
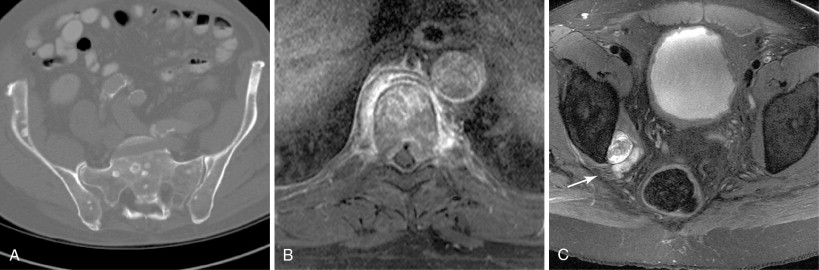
A focused physical examination should be performed prior to ablation of metastases, particularly when performed for palliation of painful metastases. In this context, the site of maximal pain or tenderness should correlate with the index lesion to be treated on cross-sectional imaging. In addition, assessment of neurologic deficits prior to the ablation should be performed. For tumors near motor nerves, frequently those located in the pelvis, preablation documentation of strength in the at-risk pathways may influence the decision to pursue ablation, alter the planned path to the target lesion, and/or improve informed consent to the risks involved in the procedure. Careful consideration of bowel and bladder continence may also be necessary to treat tumors in the pelvis ( Figure 28-3 ).
Laboratory studies
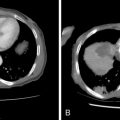
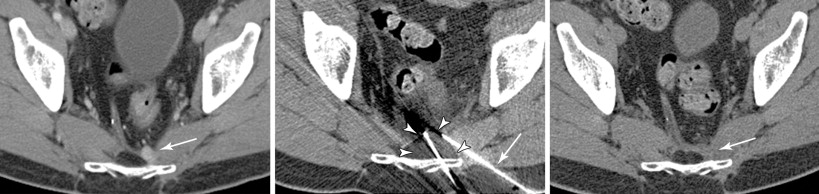
Preablation workup should include limited laboratory studies typically performed before any nonsuperficial percutaneous intervention, including platelet count and coagulation parameters. In general, platelets should number 50,000 or greater, and international normalized ratio (INR) should be 1.5 or less. Preprocedural transfusion with blood products may be required to achieve these laboratory values and minimize the risk of hemorrhagic complications. Additional laboratory studies may be helpful, depending on the primary tumor histology and clinical scenario. For symptomatic, hormone-secreting tumors, such as neuroendocrine metastases, assessment of serum or urine hormone levels (e.g., catecholamines, serotonin, 5-HIAA) allows quantitative measurement of ablation effect ( Figure 28-4 ). For tumors with elevated serum tumor markers, such as hepatocellular carcinoma (AFP), colon carcinoma (CEA), ovarian carcinoma (CA-125), or prostate carcinoma (PSA), measurement of these tumor markers before and after ablation allows assessment of the effect of the treatment and may improve detection of early tumor recurrence.
Imaging assessment
High-quality preablation cross-sectional imaging is essential for patient selection and treatment planning. The number of targeted metastases should be limited, typically between one and three lesions and very rarely greater than 5 lesions. Patients with more numerous metastases are more suitable for systemic, rather than focal, therapy, as it is difficult to localize symptoms with widespread disease. In patients referred for control of oligometastatic disease, patients should be adequately staged with imaging, often with CT of the entire torso or whole body positron emission tomography or computed tomography (PET/CT), to avoid unnecessary treatment of a few metastases in a background of additional untreated tumors. In patients referred for palliation of painful metastases, a metastasis should be present on imaging to correlate with the site of a patient’s reported pain. All types of skeletal metastases may be treated with percutaneous ablation. However, osteolytic tumors, mixed osteolytic/osteoblastic tumors, and predominantly soft tissue tumors are more technically accessible, but when necessary, osteoblastic metastases may be treated with the use of bone biopsy or drill devices.
Assessment with the imaging modality to be used for intraprocedural guidance should be performed prior to the procedure to ensure adequate visualization. Imaging should guide safe percutaneous access to the metastasis and assess nearby vital structures, particularly nerves, to be avoided or monitored during treatment. A margin of 1–1.5 cm around the target lesion(s) without involving a critical structure is ideal. If the adjacent structure falls within this margin, adjunctive procedures to displace the structure from the tumor or to monitor the structure for collateral injury are often necessary. In cases in which the distance between a critical structure and the ablation zone is expected to be narrow, the risks and benefits of the procedure should be carefully considered. A combination of imaging modalities (e.g., ultrasonography and CT) may be required for initial assessment. Although MRI is not often used for intraprocedural guidance and monitoring in most centers, it may be valuable to define the local anatomy best and subsequently use landmarks on CT or fusion-imaging for intraprocedural guidance.
Technique
Anesthesia and medications
Level of anesthesia for tumor ablation procedures depends on local preference and practice patterns and may be altered by patient preference. General anesthesia provides maximal control of a treated patient’s airway, respiration, hemodynamics, and positioning and minimizes intraprocedural patient discomfort. Conscious sedation shortens procedure time and is less expensive. Minimal sedation levels allow focused neurologic physical examination during the procedure to serve as a method for monitoring of vulnerable neural structures. Cryoablation may be possible with lower levels of anesthesia than heat-based techniques because of the relative painlessness of ice formation within the body. Tumor size, location, accessibility, and proximity to critical structures all contribute to the complexity of each case and may dictate the level of anesthesia needed for successful treatment. Conscious sedation is usually sufficient for cementoplasty.
Long-acting local anesthetics (e.g., bupivacaine, ropivacaine) administered intraprocedurally along the periosteum are frequently helpful to diminish postprocedural pain. Intraprocedural intravenous prophylactic antibiotics covering skin flora (cephazolin, 1–2 g) are recommended for percutaneous vertebroplasty and frequently administered during other percutaneous interventions, although there is no clear consensus on the need for prophylactic antibiotics for tumor ablation. In general, antiplatelet agents and anticoagulants should be held before these procedures and reinitiated 24–48 hours later.
Imaging guidance and monitoring
Appropriate imaging guidance is required for percutaneous ablation of metastases to bone and soft tissue. The modality used to guide the procedure depends on its ability to visualize the tumor of interest and associated local anatomy for device positioning and ablation monitoring. Fluoroscopy offers high spatial and temporal resolution, relatively low expense, and lower radiation exposure than CT. It is frequently used to guide vertebroplasty procedures and may be used for ethanol ablation. However, it is not sufficient for thermal ablation techniques because of limited visualization of small skeletal or soft tissue metastases and inability to monitor the ablation zone. These limitations may not be present when using angiographic c-arms with flat panel detectors capable of producing cone beam three-dimensional CT scan reconstructions. ,
Ultrasonography provides good spatial and excellent temporal resolution, is widely available, and is inexpensive. However, its use is restricted to visualization of superficial lesions with large soft tissue components. It cannot target deep lesions or those with overlying intact cortical bone, and the sound waves cannot penetrate the gas produced by heat-based techniques or ice produced during cryoablation. The gas seen during ablation does not reliably correlate with the size of the ablation zone, and only the superficial aspect of the ice ball is seen. Typically, ultrasonography is very effective as guidance during applicator placement, whereas CT and MRI are used for ablation monitoring ( Figure 28-5 ).
CT, alone or in combination with ultrasonography, is the most commonly used imaging modality for musculoskeletal ablation procedures. Its strengths include wide availability, good spatial resolution of most bone and soft tissue tumors, excellent overview of the local tumor environment and access pathway, and good temporal resolution with continuous or intermittent CT fluoroscopy techniques. Multidetector CT scanners may rapidly reconstruct images to provide multiplanar evaluation for device placement and ablation zone coverage. During cryoablation procedures, intermittent unenhanced CT scanning, usually performed every 2–3 minutes during freeze cycles, allows visualization of the growing ice ball for accurate ablation zone monitoring. Radiation exposure during CT-guided ablations should be controlled through careful dose reduction techniques.
MRI provides the highest bone and soft tissue contrast resolution. Near-real-time imaging sequences for device placement as well as temperature-sensitive sequences for thermal ablation monitoring are now available. MRI is the only imaging modality that can provide some accurate intraprocedural information regarding the volume treated with heat-based techniques. It may be used during cryoablation, showing the ice ball as an area of signal void, and is also used for guidance during focused ultrasonographic procedures ( Figure 28-6 ). However, MRI-guided procedures are often time consuming. Moreover, MRI-compatible ablation equipment is limited, and MRI scanners are not available for interventional procedures in most clinical settings.
Special devices
Bone access
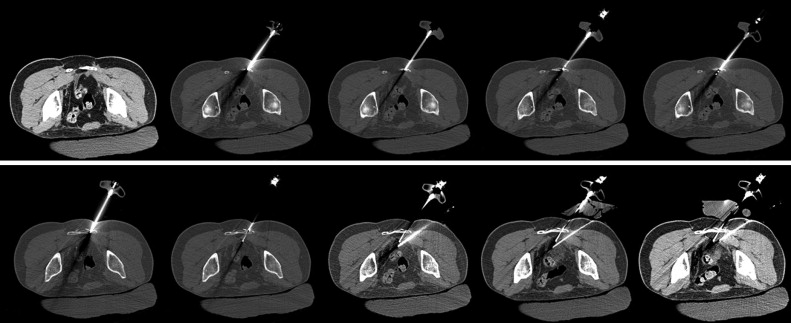
Percutaneous ablation applicators may be placed directly into metastases that are completely or predominantly composed of soft tissue, osteolytic metastases with disrupted or thin overlying cortical bone, or metastases within severely osteopenic bone. Special equipment may be required, however, to access sclerotic metastases or lesions deep to intact, thick healthy cortical bone for any percutaneous ablation procedure. Access may be obtained manually with the use of a bone biopsy kit or trocar. These are typically 10- to 14-gauge in diameter with sharply beveled or trephinated tips. Ablation device applicators may be placed in a coaxial fashion if they are of satisfactory length and diameter ( Figure 28-7 ). An automatic bone drill with drill bits or Steinmann pins may be needed in cases in which manual pressure with a bone biopsy needle is insufficient.
Radiofrequency ablation
Radiofrequency ablation (RFA) is the most widely used method of percutaneous tumor ablation. In bone, RFA was first applied to the treatment of osteoid osteomas and has become the standard of care for these benign tumors. , Application of RFA to the treatment of malignant bone tumors was originally reported in 1998 and is now routinely used. High-frequency (450–600 kHz) alternating current is delivered through a needle applicator, producing focal ionic agitation in the intra- and extracellular spaces around the exposed tip. This leads to heat generation up to lethal temperatures, between 60°C and 100°C. Protein denaturation, cell death, and coagulative necrosis occur locally. At greater temperatures, tissue carbonization and vaporization occurs, which can insulate electrical conduction and impair maximal treatment effect. To complete the electrical circuit and dissipate the deposited energy, grounding pads are placed on the skin surface, typically along the upper thighs. RF generators and applicators, called electrodes, are made by several different vendors. The electrodes are generally 17-gauge in caliber with uninsulated tips, which heat the tissue. Various strategies have been used to increase the volume of tissue ablated. Cluster electrodes, composed of three linear electrodes connected proximally, and expandable electrodes, which have multiple tines to be deployed to a varying degree by the operator, are shaped to allow larger burns ( Figure 28-8 ). In addition, some electrodes are internally cooled with chilled water, while others perfuse the tips with saline into the tissue in an effort to prevent tissue charring and resultant insulation to RF energy. Once an impedance limit (for some systems) or a target temperature of 100°C is reached, the tumor is ablated for 5–15 minutes. Tumors ≤3 cm may be treated with a single ablation, whereas larger tumors are generally treated with multiple overlapping ablations. A maximum of three electrodes may be used simultaneously with a switching generator.