Fig. 8.1
Estimated 5-year survival and recurrence rates of rectal cancer patients over time as they relate to the proportion of rectal cancer operations comprised by abdominoperineal resections
Indications
The indications for APR have evolved significantly over the past century. As noted above, due to advances in surgical technique, technology, and understanding of rectal cancer biology, fewer and fewer patients require APR to achieve adequate local control. The primary objective of rectal cancer surgery remains oncologic cure with the preservation of sphincter and sexual and urinary function as desirable secondary goals. As such, the choice of surgical procedure depends on the surgeon’s ability to achieve histologically negative proximal and distal margins and to perform a total mesorectal excision to obtain adequate radial clearance and lymph node dissection. The selection of APR over a less morbid sphincter-sparing operation is most often determined by the distal extent of disease. Inability to clear distal disease, resulting in a positive margin, is associated with a local recurrence rate approaching 40% (HR = 16.8, 95% CI 4.8–5.9) [13] and decreased 5-year survival (HR = 2.35, 95% CI 1.08–5.11) [14].
Defining the adequate distal surgical margin in rectal cancer has been the source of significant investigation over the past half century. In the 1950s, Robert Grinnell of Columbia University in New York City recommended a 5-cm distal margin for rectal cancer based on histologic examinations of distal intramural extension [15]. Subsequent studies have demonstrated that distal intramural spread beyond 1 cm occurs in less than 20% of specimens [16, 17]. And when present, distal intramural spread is associated with a poorly differentiated, aggressive cancer and portends a poor prognosis from distant failure regardless of distal margin status [16–18]. A 2-cm negative distal margin was established in the 1980s by multiple retrospective studies [19–21] and a secondary analysis of patients from the randomized National Surgical Adjuvant Breast and Bowel Project (NSABP) R-01 trial . In this study, no significant differences were identified in relative risk of local failure or relative risk of death when comparing distal margins of <2 cm, 2–2.9 cm, and >3 cm [22]. Subsequent studies confirmed acceptable and equivalent recurrence and survival rates using 2-cm distal margins [18, 23–25]. However, the 2-cm distal margin rule was largely established before the routine use of total mesorectal excision (TME) and neoadjuvant chemoradiation [26]. The most recent studies evaluating acceptable distal margins in the setting of current treatment practices have shown that microscopically negative margins, even when <1 cm, are associated with low local recurrence rates [14, 27–29]. In a meta-analysis of 3680 patients from 13 studies treated with sphincter-saving resections with or without chemoradiation , no significant difference in local recurrence rates were found among cases with a negative distal margin less than 1 cm compared to greater margins [30].
Based on these data, most specialized centers are able to apply sphincter-sparing techniques for the majority of low rectal cancers and reserve APR for malignancies with significant direct involvement of the sphincter complex or bulky tumors which preclude a negative distal margin. Certainly, in cases of impaired preoperative sphincter function, such patients may be better served by APR even if sphincter preservation is technically feasible (see Quality of Life section below).
Techniques
Principles
Regardless of operative technique, the oncologic surgical principles remain the same. The concept of total mesorectal excision (TME) , as originally described by Richard Heald in the early 1980s, includes meticulous surgical dissection of the rectal mesentery and has been shown to increase overall survival and decrease local recurrence [31]. The open operation begins with mobilization of the descending and sigmoid colon from the lateral attachments at the white line of Toldt. The colon and its mesentery are mobilized medially off the retroperitoneum as the ureter is identified and protected. The splenic flexure is not usually taken down since an anastomosis will not be created. If additional length is required, the splenic flexure may be taken down and the inferior mesenteric vein ligated near the duodenal-jejunal flexure medially. The origin of the superior rectal artery off of the inferior mesenteric artery (IMA) is identified and is ligated at this point. The corresponding point on the descending colon represents the proximal resection margin, and the colon and adjacent mesentery are divided at this level (see Fig. 8.2). For minimally invasive approaches, a medial to lateral approach is often taken to isolate the superior rectal pedicle. This then facilitates a complete submesenteric dissection prior to the division of the remaining lateral attachments of the sigmoid and descending colon. Frequently, the descending colon is not immediately divided to allow traction on the rectum during minimally invasive pelvic dissection.
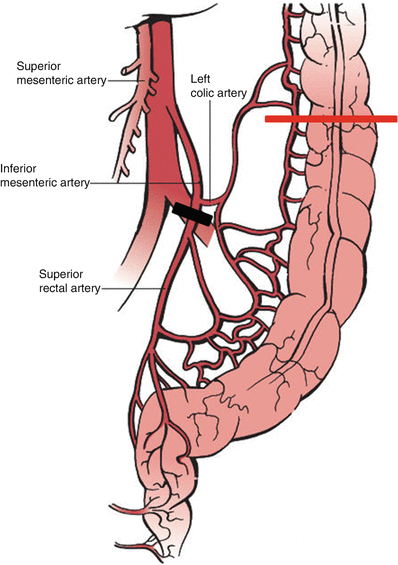

Fig. 8.2
Relevant vascular anatomy of the rectosigmoid . Ligation of the superior rectal artery at its origin off the inferior mesenteric artery preserves the left colic artery optimizing perfusion to the descending colon and eventual end colostomy. From Fischer’s Mastery of Surgery textbook, sixth ed
At this point, attention is directed toward pelvic dissection, and the patient is positioned in Trendelenburg position with the small intestines packed away for improved visualization. The rectal dissection is begun at the sacral promontory, and the retrorectal space is identified. A circumferential dissection of the rectum and mesorectum is performed as described in the chapter on total mesorectal excision. Laterally, care is taken to avoid injury to the internal iliac vein as well as the ureters at the pelvic brim. Posteriorly, care is taken to identify the hypogastric nerves as they course from the midline laterally; injury to these structures can result in retrograde ejaculation . Anteriorly, the anterior rectal wall is separated from the bladder and, in men, the prostate and seminal vesicles and, in women, the posterior vaginal wall along the fascia of Denonvillier (see Fig. 8.3). In most cases, dissection can be carried out posterior to Denonvillier’s fascia, but anterior dissection may be indicated to optimize circumferential margin for anterior-based rectal lesions.
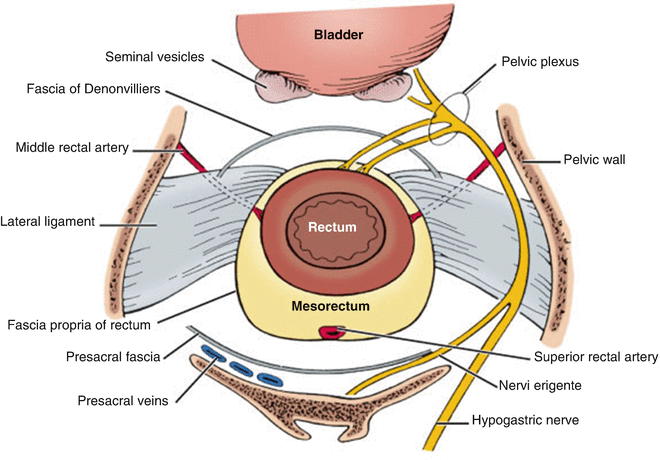

Fig. 8.3
Pelvic anatomy as it relates to the technique of total mesorectal excision during abdominoperineal resection
Circumferential rectal dissection is then carried down to the pelvic floor at the level of the levator ani muscles. The lateral attachments are divided close to the mesorectum to avoid injury to the lateral nerve plexuses which could potentially contribute to impotence in men and bladder dysfunction. At this point, a critical technical step in APR must be considered. The historical literature typically demonstrates a significantly greater degree of radial margin positivity and corresponding rate of local recurrence in APR as compared to LAR [32, 33]. It has been theorized that this may be due to inadequate wide excision at the level of the pelvic floor.
Indeed circumferential rectal margin (CRM) status is a predictor of outcome and is considered an important surgical quality indicator. Of the 455 patients who underwent APR as part of the Dutch Rectal Cancer Trial , 29.6% had a positive CRM. Location of tumor was the most important predictor of CRM with anterior tumors being the most likely to have a positive CRM when compared to laterally located tumors (44% vs. 21%, p < 0.0001) [34]. Local recurrence rates of ~22 and ~5% have been reported for patients with positive and negative CRM, respectively [35]. Positive CRM has also been associated with increased rates of distant metastasis [35]. Quirke et al. found that the combination of a negative CRM and optimal TME offers the lowest chance for local recurrence (1% at 3 years) [36].
As such, there has been greater emphasis placed on achieving wide local excision of the levator ani muscles via a “cylindrical dissection ” via what has been referred to as extralevator abdominoperineal excision (ELAPE) [37]. From a procedural standpoint, the wide division of the levator ani muscles can be achieved from either the abdominal or perineal approach. Regardless of approach, the deep dissection should maintain a cylindrical shape to avoid “waistcoating” of the mesorectum at the level of the levator ani muscles (see Fig. 8.4). ELAPE has been shown to have improved CRM at the expense of a potential increase in wound and other complications [38].
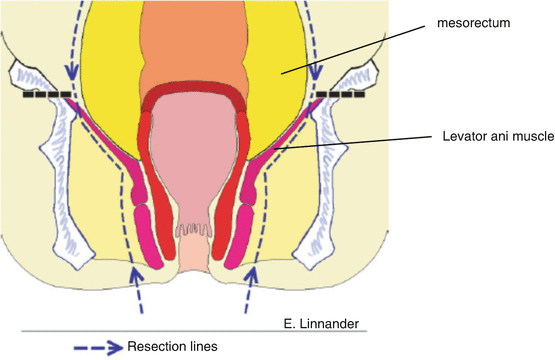

Fig. 8.4
Lines of dissection during an extralevator abdominoperineal excision (ELAPE) . Note that the levator ani muscles are divided near their insertion on the pelvis. This technique allows for a wider “cylindrical” dissection avoiding “waistcoating” of the distal mesorectum
The perineal portion of the procedure can be performed with the patient in either the lithotomy (Lloyd-Davies) or prone jackknife (Kraske) position . For the prone approach, upon completion of the abdominal portion, pelvic drains are placed, the wound closed, and the colostomy matured prior to repositioning the patient. Some authors claim that in prone position, exposure is improved along the anterior dissection plane and prostatic urethra leading to decreased operative time and blood loss [39, 40]. The lithotomy position requires no additional repositioning and offers the advantage of simultaneous access to both the abdomen and perineum, which in turn also offers the ability for a time-efficient two-team synchronous approach. Regardless, effective and safe extralevator resection is possible from either patient position and are considered equivalent [41–43].
The landmarks of the perineal resection are the coccyx posteriorly, the prostatic urethra anteriorly, and the ischiorectal fossae laterally (see Fig. 8.5). An extrasphincteric circumferential incision is made at the perineum, and an extralevator dissection continues the cylindrical shape. Posteriorly, the perineal dissection is first connected to the previously completed abdominal dissection immediately anterior to the coccyx. Subsequently, circumferential perineal extralevator dissection continues by transecting the levator ani muscles at their origin. The anterior rectal dissection is generally performed last to improve visibility and reduce chances for injury to the urethra in men and the vagina in women. Upon removal of the specimen, the perineal defect may be closed primarily or with flap reconstruction (see below) as indicated. The authors prefer the placement of a transabdominal closed suction drain. Some surgeons prefer transperineal placement; however, this has been associated with increased patient discomfort [44]. If not already completed, the midline incision is closed and the colostomy matured.
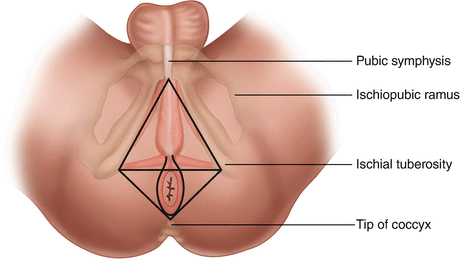

Fig. 8.5
Landmarks of the perineal dissection during abdominoperineal resection. An extrasphincteric elliptical incision is performed as illustrated. The posterior dissection proceeds anterior to the coccyx and laterally in the ischiorectal fossae. The anterior extent of the dissection is the prostate in men and vagina in women
Maturing the End Colostomy
Unlike emergent situations when a colostomy is potentially temporary and reversible, the colostomy performed at the time of APR is permanent. As such, patients may live decades following curative APR, and special care should be given to perform a technically precise and meticulous end colostomy in order to avoid both short-term and lifelong complications.
Choosing a practical and functional location of the colostomy on the patient’s abdominal wall is essential to avoid the morbidity and diminished quality of life associated with poorly functioning ostomy appliances. The left-sided location should be chosen prior to surgery taking into consideration body habitus, skin folds when sitting, and patient preferences. The colostomy should be well within reach of the patient so he/she can independently empty and change the ostomy appliance . It should be located either above or below the belt line and skin folds, and previous scars should be avoided to prevent leakage from the appliance. Preoperative education and marking provided by a trained enterostomal therapist is valuable and can help prepare the patient for this life-altering situation.
Prior to closing the abdominal fascia, a 2–3-cm circular incision is made, the skin excised, and the tissues dissected down to the fascia. A fascial defect is made through the anterior and posterior rectus sheath. The distal colon is extracted through the rectus sheath, and the muscle fibers are split, rather than transect. As the distal colon is brought through the fascia to the skin, special care must be taken to avoid twisting of the colon or avulsion of the mesentery which may result in devascularization. The fascial opening should allow a snugly fit finger in addition to the bowel and its mesentery. The bowel should be externalized without tension to a point approximately 3 cm above the level of the skin.
Once the abdominal wall and skin closure is complete, the colostomy is matured by removing the staple line from the externalized bowel. The mucosa and mesentery are assessed to ensure preserved perfusion and viability. The mucosa is secured to the subcuticular layer of the skin using interrupted 3-0 or 4-0 absorbable sutures. The bowel wall should be everted such that the colostomy protrudes 1–2 cm above the skin to facilitate placement of the ostomy appliance.
Approach
Surgical approaches include open, laparoscopic-assisted, and robot-assisted techniques. Pivotal early studies such as the COST and the MRC CLASICC trials showed that the laparoscopic approach for colon cancer is oncologically comparable to open colon surgery [45–47]. However, given the additional complexity of rectal surgery, the data showing benefits for the laparoscopic approach for rectal cancer are not as definitive. The COLOR II trial has reported on the long-term results of laparoscopic rectal cancer surgery and shown no difference in the rates of local recurrence between laparoscopic and open surgery, overall. One interesting finding in the study was the fact that the rate of CRM positivity was significantly lower in the laparoscopic arm for patients with distal rectal cancer. However, this might be explained by the notably higher than expected rate of CRM positivity in the open arm. Long-term results of the ACOSOG Z6051 and ALaCaRT trials are awaited; however, neither study could establish the oncologic non-inferiority of laparoscopic surgery in the short term. However, short-term non-oncologic benefits of laparoscopic surgery have been demonstrated, including less intraoperative blood loss (200 vs. 400 ml, p < 0.0001), shorter hospital stay (8 vs. 9 days, p = 0.036), and decreased time for return of bowel function (2 vs. 3 days, p < 0.0001) [48]. The COREAN trial randomized patients with mid-rectal cancers to laparoscopic or open surgery and showed no difference in surgical specimen quality as measured by lymph node harvest, CRM, and macroscopic quality of TME [49]. They also showed shorter hospital stay and faster return of bowel function following laparoscopic rectal surgery. Despite the current data from prospective randomized control trials, there remains great interest for considering laparoscopic surgery for low rectal cancer with the caveat that long-term oncologic equivalency to open surgery has not been established.
Robot-assisted minimally invasive approaches to rectal cancer surgery have gained popularity in recent years due to the improved instrumentation and visualization. A meta-analysis by Yang et al. evaluated 16 studies comparing laparoscopic to robotic rectal cancer surgery and found that the robotic approach had lower blood loss and conversion rates but higher costs when compared to the laparoscopic approach. The two techniques were similar in terms of complications and early markers of surgical quality including CRM and lymph node harvest [50]. Nonetheless, large-scale prospective analyses that examine both clinical outcomes and cost-effectiveness remain necessary.
Extended APR
For patients who have locally advanced, nonmetastatic primary, or recurrent tumors, multivisceral resections may offer the best chance for cure. Pelvic exenteration traditionally involves en bloc removal of the rectum along with its adjacent pelvic organs which include the bladder and prostate in men and the bladder and uterus in women. Additional extended resections may also involve partial sacrectomy or partial vaginectomy . Yamada et al. demonstrated that 5-year survival rates were higher among patients with locally recurrent rectal cancer treated by curative-intent exenteration as compared to those who underwent palliative non-oncologic resection (22.9% vs. 0%, p = 0.0065) [51]. Law et al. observed that pelvic exenteration was associated with a high risk of perioperative morbidity (54%) and an overall 5-year mortality rate of 44% [52]. Multivisceral resection including sacropelvic resection can have good oncologic outcomes with 5-year disease-free survival rates in the range of 43% [53]. The preoperative evaluation should include high-quality imaging of the pelvis. Any concern for extra-rectal sacral, prostatic, bladder, or vaginal involvement warrants further investigation with MRI.
Sacral resection may be indicated in cases of primary or recurrent rectal cancer with posterior bony extension. Tumors at or below S3 are amenable to standard partial sacrectomy. Milne et al. reviewed 100 cases and found a high complication rate (74%) with 43% of these classified as major morbidity. Neurologic complications were increased if the resection was above the S3 level [54]. Among patients from Memorial Sloan Kettering who presented for abdominosacrectomy because of recurrent disease, their complication rate was 59%, and disease-free survival was 20% at 5 years [55]. For tumors at or above S2, a high sacrectomy is possible but carries a higher risk of neurologic complication, increased morbidity, and decreased disease-free survival [56, 57].
The use of a coordinated multidisciplinary team approach is critical for the management of rectal cancer and particularly important in the setting of locally advanced primary or recurrent rectal tumors. The integrated surgical team often includes involvement of specialists from urology, gynecology, plastic surgery, and/or neurosurgery.
Use of Ureteral Stents
The routine use of ureteral stents for the identification and prevention of ureteral injury during APR is controversial. There are no randomized trials evaluating the prophylactic use of ureteral stents during colorectal cancer surgery owing to the fact that the incidence of ureteral injury is so low [58]. A recent nationwide analysis using data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample reported that among all patients who suffered an iatrogenic ureteral injury, rectal cancer was the most common indication for surgery, yet ureteral injury only occurred in 7.6 per 1000 cases during APR [59]. Comparison studies have not shown a decrease in the incidence of ureteral injuries during colorectal surgery with the use of ureteral stents [58, 60], but some authors suggest that ureteral stents allow for more apparent intraoperative recognition of an injury [61, 62]. Furthermore, ureteral stent placements are not without complications . Complications from ureteral stents include transient hematuria, urinary tract infections, hydronephrosis, and reflex anuria [63, 64]. One study of 66 patients reported that hemodialysis was required in two patients due to anuria following ureteral stent placement [65]. In addition to potential complications, ureteral stent placement adds cost and operative time to each procedure [64, 65]. It is our practice to selectively use ureteral stents in those cases when dense pelvic adhesions are expected, such as in cases of prior pelvic surgery, remote pelvic radiation (external beam or brachytherapy), or recurrent bulky disease which appears to involve one or both ureters.
Reconstruction of the Perineum
Perineal wound complications (including bleeding, infection, dehiscence, fistulae, perineal herniation, chronic pain, and delayed wound healing) occur in 16–41% of patients following APR [66, 67] and are, consequently, a major source of morbidity. These complications are exacerbated by neoadjuvant radiation, which roughly doubles the rate of perianal complications [66]. Patients with obesity and significant comorbidities are also at increased risk for perineal wound complications [67, 68]. Furthermore, depending on the location and extent of involvement, rectal tumors can invade the vagina, prostate, bladder, and/or coccyx, and en bloc resections can result in substantial soft tissue defects too large for primary closure. These issues are particularly pertinent in patients with recurrent anal cancer, as these patients almost universally receive external beam radiation as part of their primary treatment and often require wide perineal soft tissue excisions to obtain negative margins. That said, most perineal wounds following APR are amenable to primary closure; however, large perineal defects require plastic surgery reconstruction . While a complete discussion of the reconstruction techniques used is beyond the scope of this book, the four most common methods are introduced here.
V-Y Advancement Flap Reconstruction
Some perineal wounds are too large for a tension-free primary closure but do not require myocutaneous flaps to close the soft tissue defect. In these situations, a V-Y advancement flap of skin and subcutaneous tissue is a common technique to allow for a tension-free closure. This is performed by incising the skin in a wide “V” shape extending from the anterior and posterior perineal incision laterally. The soft tissue is mobilized off the underlying muscle as needed preserving perforating vessels and the flap is advanced medially until the perineal wound can be closed without tension. The remaining incisions are closed in a “Y” configuration [69].
Vertical Rectus Abdominis Myocutaneous (VRAM) Flap Reconstruction
VRAM flaps rely on the inferior epigastric artery and are created by harvesting the rectus abdominis muscle off of the posterior rectus sheath with inclusion of a vertically oriented elliptical strip of overlying fasciocutaneous tissue [70]. Often an obliquely curved overlying skin paddle extending to the costal margin is incorporated to increase length. The flap is transposed and rotated into the pelvis (see Fig. 8.6). If a partial vaginectomy was performed, a neovagina can be constructed by folding the skin paddle on itself and recreating the vaginal wall. While the preferred method of perineal reconstruction following APR by most plastic surgeons, a VRAM cannot be used in patients with a prior abdominoplasty, and may need careful consideration in a patient requiring exenteration with placement of bilateral ostomies.
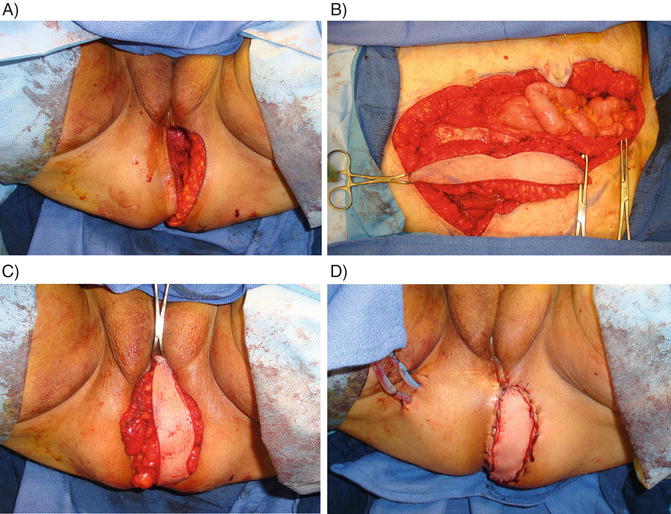

Fig. 8.6
Reconstruction of the perineal defect using a vertical rectus abdominis myocutaneous (VRAM) flap . (a) Perineal defect following abdominoperineal resection. (b) Harvested right rectus abdominis muscle with vertically oriented elliptical overlying fasciocutaneous tissue. (c) VRAM flap rotated into position to fill the perineal defect. (d) Flap sutured in place with perineal drains
Primary healing has been reported in greater than 85% of VRAM flap reconstructions with nearly 100% healing during follow-up [71–73]. The incidence of wound complications following VRAM flap reconstruction is 18–29% and includes wound infection, bleeding, partial or complete flap necrosis, disunion, vaginal stenosis, and perineal or abdominal hernia [72, 73]. Controlled studies have shown that when compared to primary closure, VRAM flap reconstruction demonstrates lower incidences of wound complications including abscesses, dehiscence, and delayed wound healing [74–76] despite the fact that VRAM flaps were used in higher-risk wounds.
Gracilis Flap Reconstruction
Gracilis myocutaneous flaps maintain their blood supply from the medial circumflex branch of the profunda femoris artery. The cutaneous flap is harvested from the medial thigh over the proximal and middle third of the gracilis muscle (see Fig. 8.7). In general, gracilis flaps are used for smaller defects or if a VRAM is unavailable [70]. Gracilis flap reconstruction results in primary healing in over 94% of patients [71] and is associated with fewer perineal complications compared to primary closure [77, 78]. In a study of locally recurrent, highly radiated cases of rectal cancer, 11 out of 24 patients who underwent APR with primary perineal closure developed a major infection requiring hospitalization and/or reoperation compared to 2 out of 16 patients following gracilis flap reconstruction [78].
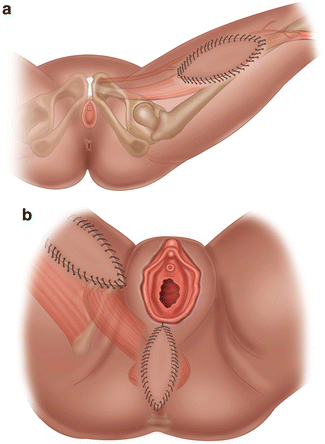

Fig. 8.7
Gracilis flap reconstruction . (a) The blood supply of the gracilis myocutaneous flap branches off the profunda femoris artery. The skin pedicle is harvested over the middle of the gracilis muscle. (b) The flap is rotated superiorly to fill the perineal defect
Gluteal Flap Reconstruction
Gluteus maximus myocutaneous flaps are based on the superior and/or inferior gluteal arteries and are rotated medially to fill the perineal defect. Perineal reconstruction using unilateral or bilateral gluteal flaps is often performed following extralevator abdominoperineal excision or extended APR where the levator muscles and/or involved adjacent organs are resected en bloc (see above) creating larger perineal soft tissue defects. In one recent report of 28 gluteal flap reconstructions, 86% of patients had complete primary wound healing, while four patients developed local wound infections or partial wound rupture [79]. Another study reported perineal wound complications in 42% of 65 patients following gluteal flap reconstruction, although 91% had completely healed by 1 year [80].
Other Reconstruction Techniques
There is evidence to suggest that the use of mobilized greater omentum to fill the pelvic dead space following APR decreases the incidence of perineal wound complications. One study analyzed the complication rates among 70 primary or myocutaneous perianal reconstructions with or without omentoplasty and found major pelvic complications occurred less frequently when an omental flap was used to buttress the perianal closure (21% vs. 61%, p < 0.01) [81]. Another study reported less perineal dehiscence following omentoplasty (5% vs. 16%, p = 0.04) [82]. In a recent meta-analysis, omentoplasty added a median of 20 min to the operation but was associated with a higher rate of primary wound healing (67% vs. 50%, p = 0.05) and a lower incidence of wound infection (14% vs. 19%, p = 0.03) [83].
Placement of biologic mesh to reinforce the pelvic floor has received some attention as an alternative to myocutaneous flap reconstruction to reduce operative time, costs, and the morbidity of flap harvest. Its use has preliminarily been supported by several small case series [84–86], but a multicenter, randomized trial is currently underway [87] comparing outcomes with and without the use of porcine-derived biologic mesh . Christensen et al. [88] compared perineal biomesh (n = 24) to gluteal flap reconstruction (n = 33) and reported higher but nonsignificant infection rates (17% vs. 6%, p = 0.26), lower rates of perineal hernia (0% vs. 21%, p < 0.01), and shorter hospital stay (9 vs. 14 days, p < 0.05) with the use of biomesh. In another small comparison study, VRAM reconstruction (n = 5) was associated with longer median operative times (405 vs. 259 min, p < 0.01), longer length of stay (20 vs. 10 days, p = 0.07), and almost double the median costs (p < 0.01) as compared to biologic mesh reconstruction (n = 10), while early complication rates were similar (80% vs. 70%, p = 0.37).
APR-Specific Complications
Overall Rates of Morbidity and Mortality
As noted previously, during its infancy, APR was associated with substantial rates of perioperative morbidity and mortality. However, as with many complex operative procedures, the evolution of surgical, anesthetic, and antimicrobial techniques has resulted in substantial improvements in outcomes. In modern series, perioperative mortality rates are generally less than 2%. Nonetheless, APR remains a relatively morbid procedure with an overall complication rate of approximately 50% [89–91].
Intraoperative Complications
Presacral Bleeding
A dreaded complication of posterior rectal mobilization is presacral hemorrhage resulting from inadvertent traversal of the presacral fascia and injury to the presacral venous plexus or sacral basivertebral veins [92]. Pollard et al. described this complication as occurring in approximately 3% of patients undergoing either APR or LAR [91]. Simple suture ligation or cautery is generally unsuccessful and may in fact exacerbate bleeding [93]. Consequently, over the years, a variety of operative techniques have been described including compression methods (e.g., sterile thumbtacks [93], tamponade with saline bag [94], or tissue expander [95]), use of hemostatic agents (e.g., fibrin, cyanoacrylate glue [96], rectus abdominis muscle welding [97]), and endoscopic stapling devices [98]. Despite the availability of numerous such technologies, it must be emphasized that in the face of patient instability, packing of the pelvis allowing patient stabilization with interval return to the OR remains a sometimes necessary and highly sound approach .
Urethral Injury
Inadvertent injury to the membranous portion of the urethra can occur in males during anterior perineal dissection during APR. In older series, it has been reported that this complication may occur in 1–5% of cases [99–101]. Close attention and palpation of the urinary catheter within the urethra can help to avoid this complication. Fortunately, urethral injury can often be recognized immediately by visualization of the urinary catheter in the dissected field. This can often be managed successfully by primary repair and/or prolonged urethral stenting and drainage with a urinary catheter. Unsuccessful management or unrecognized injury may result in urinoma and/or urethrocutaneous fistula to the perineal wound which may ultimately require more complex reconstructive techniques [102].
Ureteral Injury
It is estimated that surgical procedures involving the colon and rectum account for approximately 5–15% of iatrogenic ureteral injuries [102]. Among colorectal procedures, APR is the most commonly associated with ureteral injury with frequencies reported as high as 5% [99]. Careful visualization of the left ureter is critical prior to transection of the colonic mesentery. As discussed previously, ureteral stenting can facilitate the identification of the ureters and also of an injury but generally do not prevent ureteral injury. The use of ureteral stenting can be performed at the discretion of the surgeon taking into account factors such as reoperative surgery, complexity, uncertain anatomy, and experience. Mechanisms of ureteral injury include laceration and/or transection, ligation, devascularization, and thermal injury. Suspected ureteral injury can be diagnosed intraoperatively with intravenous injection of colored dyes such as methylene blue or indigo carmine and observation for extravasation. Subsequent management depends on timing of diagnosis as well as the extent and anatomic location of the injury [102]. For injuries to the middle third of the ureter, the preferred method of repair is a spatulated ureteroureterostomy over a ureteral stent. Occasionally, ureteroneocystostomy is required with the aid of a psoas bladder hitch or Boari flap [102]. Ureteroneocystostomy techniques are the procedures of choice for the more common distal one third ureteral injuries [102].
Vaginal Injury
During APR in women, posterior vaginal defects can occur as a result of planned resection for directly invading anterior-based rectal tumors or by inadvertent injury during anterior perineal dissection. Smaller defects may be closed by primary repair in a tension-free fashion. However, it should be recognized particularly in cases treated with neoadjuvant radiation that breakdown of the repair may occur. This, in turn, may lead to vaginal stenosis, scarring, and in some cases vaginocutaneous fistula to the perineal wound or in association with persistent perineal sinus [103]. Larger defects are best managed with myocutaneous flap reconstruction as previously discussed with VRAM flaps being most preferable [73].
Perineal Wound Complications
Short Term
Immediate nonhealing of the perineal wound represents a spectrum of clinical significance ranging from superficial wound separation to pelvic abscess and sepsis and thus requires different management strategies [104]. Despite general improvements in surgical outcomes, postoperative perineal wound healing complications following APR remain a significant problem with rates ranging from 14% to as high as 50% [83, 105, 106].
Perineal wound complications are associated with substantial additional medical costs as a result of increased length of stay, hospital readmission, and home nursing care needs [104]. Additionally, Hawkins et al. noted by multivariate analysis that perineal wound dehiscence following APR was associated with a 1.7 times increase in the adjusted risk of death [107].
Risk Factors
Extirpation of the rectum and anus (and other pelvic organs for exenteration) results in a large dead space that is fixed by the surrounding bony pelvis. This cavity facilitates the accumulation of fluid and/or hematoma that increases the risk of wound leakage/infection, pelvic abscess, and wound sinus tracts. The ELAPE approach (as compared to conventional APR) and the use of neoadjuvant radiation therapy are generally associated with an increase in perineal wound complications [37, 105, 106, 108]. Indications for surgery such as malignancy and inflammatory bowel disease contribute to increased rates of nonhealing, while additional patient-specific risk factors include diabetes mellitus , anemia, obesity, and smoking [68].
Prevention
As noted, numerous approaches have been described with varying degrees of success to help mitigate the issue of nonhealing and/or infection of the perineal wound and its subsequent sequelae. In addition to reconstructive options cited above, additional maneuvers include closed suction drainage [109, 110] and pelvic placement of a gentamicin-impregnated collagen sponge [111]. Notably, the historical practice of closing the pelvic peritoneum has been associated with delayed wound healing presumably as a result of creating a significant closed pelvic dead space [112]. The addition of irrigation to closed suction drainage has been shown not to add any additional benefit [113].
Where possible, it is the authors’ practice to employ primary closure with omental pedicle flap placement in combination with transabdominal closed suction drainage. Flap closure is generally reserved for larger or extended defects (e.g., exenteration, posterior vaginectomy) particularly in the setting of presurgical radiation treatment (e.g., advanced anal squamous cell cancer).
Management
Superficial and uncomplicated wound separation may be treated with simple packing with normal saline wet-to-dry dressings. Occasionally excessive granulation tissue may require topical treatment with silver nitrate. Traditional initial management of complete perineal wound dehiscence has consisted of debridement of devitalized tissue and wet-to-dry dressing changes. However, in the setting of abundant fibrinous exudate and/or low-grade infection, the use of one fourth strength Dakin’s solution or silver-impregnated preparations may be used [104]. With these routine wound care measures, it is estimated that almost 90% of wounds will heal within 6 months [104]. It is important to emphasize that complete dehiscence does require careful examination to ensure that there is no evidence of small bowel evisceration which may require urgent operative intervention.
Recently, the use of vacuum-assisted closure (VAC) devices has become relatively commonplace to accelerate healing by secondary intention particularly for large abdominal wounds [114]. The use of VAC dressings for perineal wounds following APR has been described [115, 116]; however, the perineum is inherently a difficult anatomic location to maintain an airtight seal necessary to allow for the application of suction. The authors have found VAC therapy for perineal wounds to be highly effective but have noted that success depends on the involvement of a dedicated and experienced wound care team.
Fever and pelvic pain with or without perineal wound drainage may be a manifestation of a pelvic fluid collection and/or abscess and should prompt CT imaging. Pelvic abscesses are most often treated by placement of percutaneous CT-guided drainage catheters and broad-spectrum antibiotics. Occasionally, in the acute setting, narrow-mouthed sinus tracts from the perineal wound may require operative intervention to enlarge the wound opening to allow for adequate drainage and more effective wound packing [104].
Long Term
Persistent Perineal Sinus
A perineal wound that remains unhealed for more than 6 months after surgery is defined as a persistent perineal sinus (PPS) and may occur in up to 30% of patients undergoing APR for rectal cancer. Development is often associated with preceding sepsis (particularly in the setting of neoadjuvant radiation) with subsequent chronic inflammation and fibrosis which in turn contribute to reduced tissue oxygenation and poor healing capacity [117]. The sinus is typically a long fibrous tract lined with granulation tissue with a narrow external opening at the perineal wound and often extending into a presacral cavity. PPSs can be associated with pain and foul-smelling and/or bloody discharge. Evaluation can include sinography, CT scan, and/or MRI to define the sinus anatomy and to exclude underlying causes such as undrained abscess, foreign body, recurrent cancer, or perineal enterocutaneous fistula [117].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree