Population-Based Surveillance for Bacterial Infections of Public Health Importance
1 University of Pittsburgh Graduate School of Public Health and School of Medicine, Pittsburgh, PA, USA
2 Centers for Disease Control and Prevention, Atlanta, GA, USA
Introduction
The Hungarian physician Ignaz Semmelweis monitored the incidence of Kindbettfieber, or childbed fever, at two divisions of the Vienna Maternity Hospital in the 1840s [1]. Through this surveillance and a series of other observations, he concluded that the higher rates of childbed fever among women delivering their babies in the physicians’ division was a result of inadequate hand washing among physicians conducting autopsies before delivering babies. Semmelweis postulated that “during the examination of gravidae, parturients, and puerperae, the hand contaminated by cadaveric particles is brought into contact with the genitals of these individuals, and hence the possibility of absorption…into the vascular system of these individuals is postulated, and by this means the same disease is produced in these puerperae.” This statement is remarkable because Semmelweis put forth this hypothesis before the discovery of bacteria; childbed fever is believed to have been caused by streptococcal species [2]. In 1847, Semmelweis established a controversial hand washing policy using chlorinated water that led to a decline in the incidence of childbed fever. Thus, Semmelweis established surveillance for a syndrome caused by a serious invasive bacterial disease, designed an intervention to reduce the incidence, and documented the positive impact of the intervention. The pioneering work of Semmelweis reminds us that surveillance is “information for action” and has as its ultimate goal the reduction of morbidity and mortality. Or, in Semmelweis’ words, “My doctrines exist to rid maternity hospitals of their horror, to preserve the wife for her husband and the mother for her child.”
The Active Bacterial Core surveillance (ABCs) network and its predecessor have been examples of using surveillance as information for action for over 20 years. ABCs has been used to measure disease burden, to provide data for vaccine composition and recommended-use policies, and to monitor the impact of interventions. ABCs data have been widely disseminated in the peer-reviewed scientific literature, and ABCs exemplify use of data to prevent the diseases targeted by surveillance.
In this chapter, we describe the history of ABCs and its methodology; give examples of how ABCs has been used to make advances in the prevention of three ABCs pathogens: group B Streptococcus (GBS), Streptococcus pneumoniae, and Neisseria meningitidis; and discuss challenges and opportunities.
History of ABCs
In 1986, to address questions about the risk of toxic shock syndrome (TSS) associated with the contraceptive sponge, the Centers for Disease Control (CDC) established active surveillance for TSS in Los Angeles County and in Missouri, New Jersey, Oklahoma, Tennessee, and Washington [3,4]. TSS is a serious, toxin-mediated illness caused by Staphylococcus aureus. Previously, TSS surveillance in the United States had been passive [5]. At the same time, active surveillance was also established for invasive infections caused by Haemophilus influenzae, Neisseria meningitidis, GBS, and Listeria monocytogenes in the same geographic areas.
This active surveillance system was used to determine the effectiveness of H. influenzae type b polysaccharide [6,7] and conjugate vaccines [8] in young children and to assess the cost-effectiveness of different approaches to preventing GBS disease in newborns [9]. This system was the precursor of ABCs, which started at CDC in 1995 under the Emerging Infections Program (EIP) as part of the agency’s strategy to address the worldwide threat of emerging infectious diseases. ABCs was developed to fulfill key components of the strategy, which included conducting surveillance and applied research activities and evaluating public health interventions related to infectious diseases that were viewed as an increasing threat to the public’s health [10]. In the 1970s, GBS became the leading cause of serious bacterial infections in neonates [11–13]. In the 1980s, group A Streptococcus (GAS)–associated necrotizing fasciitis and TSS were increasingly recognized [14–16]. In the 1990s, multidrug-resistant Streptococcus pneumoniae was emerging in the United States [17,18] and vaccines to prevent S. pneumoniae, H. influenzae, and N. meningitidis, had recently been developed or were under development. Active, laboratory, population-based surveillance for these five pathogens, which persist as major causes of community-acquired invasive bacterial infections, continues today. Surveillance for invasive methicillin-resistant Staphylococcus aureus (MRSA), which had long been recognized as an important healthcare-associated pathogen, began at three ABCs sites in 2001 and later expanded to nine sites because it emerged as an important cause of community-acquired infection [19]. Surveillance for L. monocytogenes was transitioned between 1999 and 2003 to the FoodNet component of the EIP, which conducts active surveillance for foodborne pathogens.
ABCs has continuously evolved to address challenging questions posed by the six pathogens (H. influenzae; GAS, GBS, S. pneumoniae, N. meningitidis, and MRSA) and other emerging infections. Through basic surveillance and special studies, ABCs has served as a platform to track the impact of pneumococcal and meningococcal conjugate vaccines on invasive infections [20–22]. Data have been used in developing and assessing the potential impact of GAS, GBS, MRSA and serogroup B meningococcal vaccines [23–26]. ABCs has also been used to develop and evaluate guidelines for the prevention of perinatal GBS disease [27–29]. Trends in antimicrobial resistance, including reductions in penicillin non-susceptible invasive S. pneumoniae disease in the era of vaccination [30] and increases in GBS resistance to clindamycin and erythromycin [31], have been tracked through ABCs. ABCs analyses have helped to shed light on risk factors and racial disparities associated with invasive bacterial diseases [32–35]. In addition to surveillance for invasive infections, surveillance for infections caused by Bordetella pertussis and other Bordetella species and by Legionella species were added in 2011. Despite a successful vaccine program, pertussis remains endemic to the United States and outbreaks continue to be recognized. The objective of enhanced pertussis surveillance through the ABCs program is to improve case ascertainment and epidemiologic and laboratory data collection beyond what is obtained through national passive surveillance. From 2000 to 2009, age-adjusted incidence rates of diseases caused by Legionella increased 170% from 0.40 to 1.08 per 100,000 persons according to national passive surveillance [36]. Through active legionellosis surveillance, ABCs hopes to improve population-based estimates of disease and to better characterize its clinical course and outcomes.
ABCs Sites and Infrastructure
ABCs is a collaboration among CDC, state health departments, and academic institutions that are part of the EIP network. Originally established in 1995 at four sites (California, Connecticut, Oregon, and Minnesota), ABCs now operates at ten sites with the additions of Georgia, Maryland and New York (1997), Tennessee (1999), Colorado (2000), and New Mexico (2003). These sites represent wide geographic diversity and approximately reflect the race and urban-to-rural mix of the U.S. population [37]. Currently, the population under surveillance is 19–42 million and varies by pathogen and project. Surveillance for the least common pathogens (N. meningitidis, H. influenzae) is conducted over the entire surveillance area, but catchment areas for more common pathogens, including GBS and GAS (catchment area: ∼32 million), S. pneumoniae (catchment area: ∼30 million) and MRSA (catchment area: 19 million), are more restricted. For certain projects that require larger case counts, sites outside of the EIP network may collaborate, using the same study protocol. For the meningococcal vaccine effectiveness study, for example, additional counties outside ABC areas in three states (California, Colorado, and New York) and nine non-ABCs states (Maine, Massachusetts, Michigan, New Hampshire, Pennsylvania, South Carolina, Texas, West Virginia, and Wisconsin) were added to cover approximately 54% of the United States population.
ABCs Methods
The ABCs Steering Committee comprised of the principal investigators from each site and representatives from CDC, the Infectious Diseases Society of America (IDSA), the American Society of Microbiology (ASM) and the Council of State and Territorial Epidemiologists (CSTE) set the general scientific direction and track scientific progress of ABCs activities. For the six core pathogens, the objectives are (1) to determine the incidence and epidemiologic characteristics of invasive disease in geographically diverse populations in the United States through active, laboratory, and population-based surveillance; (2) to determine molecular epidemiologic patterns and microbiologic characteristics of isolates collected as part of routine surveillance in order to track antimicrobial resistance; (3) to detect the emergence of new strains with new resistance patterns and/or virulence and contribute to development and evaluation of new vaccines; and (4) to provide an infrastructure for surveillance of other emerging pathogens and for conducting studies aimed at identifying risk factors for disease and evaluating prevention policies.
For routine ABCs surveillance, a case of invasive bacterial disease is defined as isolation of H. influenzae, N. meningitidis, GAS, GBS, S. pneumoniae, or MRSA from a normally sterile anatomic site in a resident of one of the surveillance areas. A normally sterile site is defined as a portion of the human body in which no microorganisms are found in a healthy state. Every clinical laboratory that routinely processes specimens from residents of a surveillance area is contacted for case identification. At least yearly audits of reporting laboratories are conducted to ensure that all cases are being captured. Once a case patient is confirmed to meet the ABCs case definition, a case report form is completed and an isolate from the first positive culture is collected for most core pathogens (a convenience sample of MRSA isolates is collected). Data collected for the case report form originate from the medical record and include information on patient demographics, clinical course (i.e., length of stay, intensive care admission), outcome (survived or died), infection type, infection site, underlying conditions and vaccination history. Isolates are tested for serotype or serogroup and antimicrobial susceptibility at CDC and other reference laboratories. Aliquots of over 75,000 S. pneumoniae, GA, and GBS isolates are currently stored in an isolate bank that is accessible to ABCs partners and external researchers by request [38]. A representative sample of MRSA isolates is deposited at the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA).
Examples of Use of ABCs Data for Specific Pathogens
Antimicrobial Chemoprophylaxis to Prevent Early Onset GBS
Invasive GBS infection in infants is a serious and often devastating cause of bacteremia, pneumonia, and meningitis. GBS commonly colonizes the genitourinary tract of women and may be transmitted from mother to infant during the perinatal period. Antibiotic prophylaxis during labor was shown to prevent early onset GBS [39], defined as GBS infection occurring in the first week of life. Beginning in 1996, a series of recommendations for prevention of early onset GBS infection using intrapartum prophylaxis were issued by the CDC [29,40,41]. ABCs data on disease burden have been used as a rationale for the national chemoprophylaxis guidelines, to monitor its impact, and to conduct studies that have led to program improvements.
In the early 1990s, the American Academy of Pediatrics (AAP) and the American College of Obstetricians and Gynecologists (ACOG) published separate and somewhat different recommendations for approaches to prevention of early onset GBS infection [42,43]. In 1996, the CDC, along with representatives from ACOG and the American Academy of Pediatrics, published consensus guidelines recommending that all women undergo rectal/vaginal culture for GBS at 35–37 weeks of gestation and that antibiotics be administered to GBS-positive women [40]. An alternative approach of providing antibiotic prophylaxis to women with certain risk factors, such as duration of rupture of membranes ≥18 hours, delivery at <37 weeks gestation, intrapartum fever, previous delivery of an infant with invasive GBS infection, or presence of GBS bacteruria during pregnancy was also deemed acceptable. These two prevention strategies had not been directly compared to each other. To address this issue, a cohort study of 5144 births, including 312 that resulted in early onset GBS infection, was conducted at eight ABCs sites. The incidence of early onset GBS infection was less than half (relative risk of 0.48, 95% confidence interval 0.36–0.60) among infants of women screened by GBS cultures as compared to infants whose mothers were assessed based on risk factors [44]. This finding was incorporated into subsequent guidelines in 2002, when CDC recommended that all women have vaginal/rectal screening for GBS colonization performed at 35–37 weeks’ gestation to identify women who should receive intrapartum antibiotic prophylaxis (IAP). Overall, ABCs has documented an approximately 80% reduction in the incidence of early onset GBS infection since implementation of these recommendations, which is remarkable for an intervention that does not involve a vaccine (Figure 7.1).
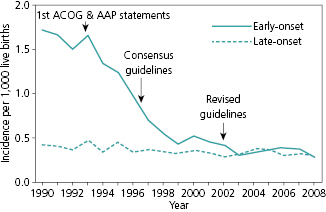
In a subsequent study conducted at 10 ABCs sites, adherence to the guidelines was assessed by review of the medical records for 254 births that resulted in GBS infection and 7437 births that did not [28]. The rate of screening for GBS during pregnancy increased substantially between 1998–1999 and 2003–2004, from 48% to 85%. Failure to screen mothers for GBS during pregnancy occurred in 13% of the deliveries that resulted in GBS infection. In addition, 61% of mothers delivering term infants with GBS infection had tested negative for GBS during pregnancy. This latter finding suggests that the screening approach, although superior to the risk-factor approach, suffers from a lack of sensitivity for detecting women at risk of having infants with GBS infection. In 2010, the guidelines were further refined to provide guidance on appropriate laboratory methods, revised algorithms for women with threatened preterm delivery, an algorithm to enhance appropriate antibiotic selection for women who are allergic to penicillin, and a revised neonatal management algorithm [41]. ABCs continues to monitor antibiotic resistance in invasive GBS isolates.
With the large declines in early onset GBS infection, there is recognition that additional interventions will be required to address the remaining burden of early onset disease and to address late onset disease, which occurs from 7 to 89 days of age and whose incidence has not declined over the past several decades. In that regard, several experimental GBS vaccines are in development. Characterization of ABCs GBS isolates has provided useful information for the formulation of these vaccines [24,45,46]. In addition, ABCs has documented a large and increasing burden of invasive GBS infection in adults, many of whom have underlying medical conditions [46]. It is conceivable that the vaccines that are being developed for infant disease could also be useful for prevention of adult disease. However, whether these vaccines would be sufficiently immunogenic and efficacious in the elderly and persons with chronic medical conditions is not known.
Invasive Pneumococcal Disease Surveillance and Vaccine Effectiveness Studies
S. pneumoniae is a leading cause of pneumonia, bacteremia, meningitis, and otitis media in the United States and globally. Pneumococcal strains can be characterized according to the composition of the polysaccharide capsule; there are currently 93 known serotypes. A 23-valent pneumococcal polysaccharide vaccine was licensed in 1983 and is recommended for adults and children with certain medical conditions. The 23 serotypes covered by this vaccine account for approximately 85% of adult invasive pneumococcal disease. In 2000, a 7-valent pediatric polysaccharide-protein conjugate vaccine (PCV-7) was licensed and recommended for use. The pivotal pre-licensure trial suggested high vaccine efficacy against invasive disease with a primary series at 2, 4, and 6 months of age and a booster dose at 12–15 months [47].
Following licensure of PCV7, ABCs documented dramatic changes in the epidemiology of invasive pneumococcal disease (IPD). First, as expected, there was a large decline in the incidence of IPD in children <5 years old [48,49]. For example, by 2007 the reductions in vaccine serotype IPD in this age group were nearly 100% (Figure 7.2) [48]. Second, the decline in the incidence in this age group was larger than expected based on vaccine coverage in the population. This suggested that herd protection was playing a role, that is, unimmunized children were protected through reductions in nasopharyngeal carriage of vaccine serotype S. pneumoniae among children who had been immunized. Third, there were substantial reductions in vaccine serotype IPD among adults, again suggesting herd protection (Figure 7.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


