Public Health Surveillance for Tuberculosis
Centers for Disease Control and Prevention, Atlanta, GA, USA
Introduction
Tuberculosis (TB) occurred in humans as early as 9000 years ago [1]. Surveillance for TB, although it started earlier than for many other diseases, is a relatively recent public health activity. The World Health Organization (WHO) estimates that 8.7 million new cases of TB and 1.4 million deaths from TB occurred in 2011 worldwide [2]. In the United States the first strategy of TB control is the prompt detection and reporting of TB cases [3]. A core responsibility of public health agencies is to assess the extent and character of TB by collecting and analyzing epidemiologic data. TB surveillance data enable public health officials to describe morbidity and mortality, monitor trends in TB incidence, detect potential outbreaks, and define high-risk populations. Since 1953, Centers for Disease Control and Prevention (CDC) has maintained a standardized national TB surveillance system focused on identifying incident TB cases and reporting TB incidence counts, rates, and case follow-up information on an annual basis.
The goal of this chapter is to introduce the reader to the epidemiology of TB and the national TB surveillance system used in the United States. The history of TB surveillance, the TB case definition, the rationale for collection of specific data, the data evaluation, and reporting will be discussed. Additionally, laws pertaining to TB reporting will be covered briefly.
TB is most commonly caused in humans by Mycobacterium tuberculosis (MTB) and is usually spread via airborne particles, most commonly by coughing, sneezing, talking, shouting, laughing, and singing. Other species in the Mycobacterium tuberculosis complex (Mycobacterium bovis and Mycobacterium africanum) can also cause disease [4]. Cough is the most common symptom in patients with TB; other frequent findings include lethargy, weight loss, fever, and night sweats.
TB infection can occur anywhere in the body; however, most disease occurs in the lungs. If the disease affects the lungs, symptoms can include coughing, chest pain, and coughing up blood. Viable mycobacteria bacillus can persist in the body for years as an inactive infection, a condition referred to as latent TB infection (LTBI). Although persons with LTBI have no symptoms, they are at risk for the eventual development of active disease. In general, persons with active TB (or TB disease) are symptomatic and can transmit the infection to others. According to the 1999–2000 National Health and Nutrition Examination Survey [5], more than 11 million persons have latent TB infection; and WHO estimates that one of every three individuals worldwide is infected with TB [6]. An estimated 5–10% of persons with LTBI in the general population will eventually develop active TB disease. Persons with latent infection who are immune suppressed for any reason are more likely to develop active disease. It is estimated that people infected with human immunodeficiency virus (HIV) are 21–34 times more likely to progress from latent to active TB disease than those without HIV infection [7], and they are more likely to die without treatment [8]. The advancement of latent TB infection to TB disease is dependent upon the degree of immunodeficiency. Those with a higher CD4 count (>350 cells/ μL) will present with TB symptoms that resemble those of individuals without HIV infection. Those with a lower CD4 count (<200 cells/μL) may not have symptoms of active TB disease and, once diagnosed, are more likely to have disseminated TB [9]. CDC began collecting information on HIV testing for TB cases in 1993 in response to a resurgence of TB from 1985 to 1992 [10,11]. In 1993, the prevalence of reported HIV-infection among TB cases was 15% although only 30% of cases were tested for HIV. By 2010, the percentage of all TB cases tested for HIV was 65% and the prevalence of coinfection was 6% [4].
Persons with TB disease can be treated with a 6- to 12-month course of multidrug therapy; however, some cases are caused by organisms that are resistant to these medications. Multidrug-resistant tuberculosis (MDR-TB), as defined by WHO, is TB that is resistant to at least two of the first-line drugs used to treat the disease (e.g., isoniazid and rifampin) [12]. MDR-TB complicates public health efforts to control disease. Some cases of MDR-TB are caused by organisms that are resistant not only to first-line antibiotics but also to the best second-line drugs—fluoroquinolones—and at least one of three injectable drugs. This subtype of MDR-TB, known as extensively drug-resistant TB (XDR-TB) [13], occurs very rarely in the United States. Fewer than six cases of XDR-TB have been reported annually for the years 2003–2009, whereas approximately 100–130 cases of MDR-TB were reported each year during the same time period [4].
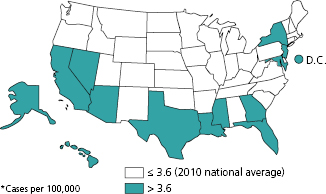
From a global perspective, the United States is considered a low morbidity and mortality country for TB. In 2010, the national annual incidence rate for TB was 3.6 per 100,000 persons with 11,182 reported cases of TB (Figure 14.1) [4]. Only 13 states and the District of Columbia had incidence rates above the national rate. In 2009, 547 persons died of TB disease in the United States, representing a rate of 0.2 deaths per 100,000 persons [4].
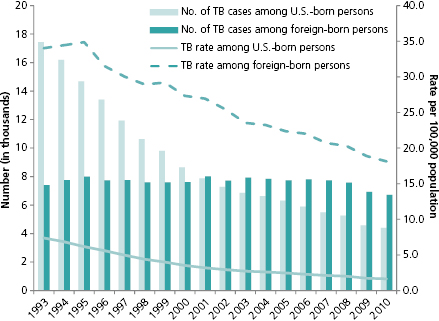
Foreign-born cases (those born outside the 50 states, District of Columbia, Guam, Puerto Rico, U.S. Virgin Islands, American Samoa, or those not born in the United States or territories and not of parents who are U.S.-born) were 29% of all reported TB cases in the United States in 1993, when the birth origin of TB cases was first collected for surveillance (Figure 14.2). By 2002, foreign-born cases were over 50% of all reported cases (51%); and in 2010, that proportion reached 60%. The birth country of origin and the number of years that foreign-born patients have resided in the United States are important monitoring indicators for TB control. Since 1991, CDC has required all immigrants and refugees traveling to the United States to be screened for TB [14]. In 2007, the technical instructions accompanying this policy were updated to include sputum culture on suspect cases, drug susceptibility testing, and treatment using directly observed therapy until the patient has completed treatment before allowing them entry into United States [15]. Implementation of the updated instructions is being phased in and may help reduce the number of foreign-born patients with TB disease entering the United States [16].
Other risk factors for TB include residence in correctional and long-term care facilities, homelessness, drug and alcohol abuse, and unemployment. In 2011, the prevalence of homelessness, residing in a correctional facility or residing in a long-term care facility among reported TB cases over age 15 was 5.8%, 4.3%, and 2.3%, respectively. Among reported TB cases over age 15, the prevalence of excess alcohol use was 12.4%; injection drug use, 1.5%; and for non-injection drug use, 7.6% [4].
Laboratory Detection of Mycobacterium tuberculosis
Laboratory detection of tuberculosis must distinguish between disease and infection. The Mantoux tuberculin skin test (TST) and the interferon-gamma release assay (IGRA) both detect infection but not TB disease. For the TST, purified protein derivative (PPD), an antigen of MTB, measures the delayed hypersensitivity reaction to MTB. It requires at least two patient visits—one to administer the test and another to read the reaction 48–72 hours later. The success of the TST depends on a patient’s intact immune function to enable reaction to the antigen and the ability of the health professional to properly read the result. The IGRA identifies the presence of MTB infection by measuring the immune response to the TB proteins in whole blood. A blood sample is required, and the test requires one patient visit to a health-care provider. Both tests have varying sensitivity, and special guidelines should be considered depending on the risk level of the patient being tested [17–19].
For many years, the mainstay of laboratory diagnosis of tuberculosis disease was detection of mycobacteria in a sputum or tissue specimen by acid-fast bacillus (AFB) stain or by culture. Although results for AFB smears can be available within 24 hours, a positive result does not rule out disease caused by nontuberculosis mycobacteria (NTM) or other acid-fast organisms, and many patients are AFB-smear negative but have TB disease. Culture-positive specimens, which can distinguish between NTB and MTB complex, allow for antimicrobial resistance testing (drug susceptibility testing) and genotyping of the MTB organism. In recent years molecular techniques for detecting MTB in a clinical specimen, detecting drug resistance, and defining the origin of the infecting mycobacteria have been developed. Direct detection of genetic material unique to MTB complex can be performed on cultured or fresh specimens using nucleic acid amplification tests (NAATs); these test allow diagnosis of MTB in fresh specimens within 24 to 48 hours [20], enabling the physician to begin treatment with anti-TB medications promptly. In some commercially available or laboratory developed tests, NAAT can also be used to detect genes associated with drug resistance. Traditional culture-based tests for detecting drug resistance involve culturing of the organism in liquid or solid media and can take several weeks to several months to complete. NAAT is still followed up with traditional detection methods in liquid or solid media culture; however, a patient may be started on the anti-TB medications to which their infection is susceptible long before the traditional methods confirm resistance [21].
In addition to diagnosis of TB, molecular characterization of MTB is being used to define the origin of infection. Genotyping techniques are used to determine the genetic makeup of the MTBC DNA. This can aid in identifying the genetic links between two or more infected patients. If the specific genotype matches other infections in the community, health workers can know whether the infection was recently transmitted and can plan appropriate contact tracing to find other infected individuals [22]. Universal genotyping of culture-positive TB specimens started in 2004 in the United States. By 2010, slightly over 88% of reported culture-positive TB cases were being genotyped [23].
TB Case Verification Criteria
The United States employs five separate hierarchical verification criteria for TB cases, which are based on laboratory confirmation or clinical criteria (Table 14.1). Laboratory criteria are based on confirmation either by isolation of M. tuberculosis complex from culture, by identification of microscopic AFB on a specimen smear, or by identification of M. tuberculosis complex DNA by NAAT from a clinical sample. AFB culturing of respiratory or tissue samples is the gold standard for confirmation of tuberculosis During 2010-2011, about 75% of TB cases in the United States have been diagnosed by culture, 1% by NAAT, and 1% by AFB positivity. All three methods require well-equipped laboratories with trained and certified laboratory personnel. Most state health departments have full-service certified TB laboratories or contracts with commercial laboratories. Local county public health laboratories and hospitals may have AFB smear capabilities and limited culture or molecular facilities for identifying M. tuberculosis. Many state TB programs now require that all reporting entities send clinical samples from suspect TB cases to their respective state public health laboratories.
Table 14.1 National TB Surveillance System TB Case Classifications.
Source: Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, CDC, October 2012
| Hierarchy | Criteria | Definition |
|---|---|---|
| 1 | Laboratory confirmation
| Isolation of M. tuberculosis complex from a clinical specimen |
| 2 | Laboratory confirmation
| Demonstration of M. tuberculosis complex from a clinical specimen by NAAT |
| 3 | Labaoratory confirmation
| Demonstration of AFB in a clinical specimen when a culture has not been or cannot be obtained or is falsely negative or contaminated |
| 4 | Clinical confirmation | Must meet all the following criteria:
|
| 5 | Provider diagnosis | The patient doesn’t meet any of the above criteria but the provider believes the patient has a TB diagnosis. |
In the absence of laboratory confirmation of M. tuberculosis from a clinical specimen, physicians can confirm a TB case based on clinical criteria (Table 14.1). Patients who have evidence of TB infection based on a positive TB skin test result or positive interferon gamma release assay (IGRA) for M. tuberculosis, who have signs and symptoms compatible with TB (abnormal chest radiograph or abnormal chest computed topography scan or other chest imaging study) or clinical evidence of current disease, who are on treatment with at least two antituberculosis medications, and who have completed a diagnostic evaluation can be clinically confirmed. If all these criteria are absent but the provider still believes the patient has TB, the patient can be confirmed as a clinical TB case by provider diagnosis.
History of Tuberculosis Surveillance in the United States
The scourge of TB in the United States was well known before there was any attempt to develop a national TB surveillance system. Prior to Robert Koch’s 1882 discovery of the bacterium responsible for TB, however, the medical establishment viewed tuberculosis as hereditary and inevitable [24]. In the nineteenth century, the most common index used to measure the effect of tuberculosis on the U.S. population was mortality data. However, reporting of that data was often limited and collected inconsistently across cities and states. During this time, the state of Massachusetts maintained a registry of deaths that was considered to be a reliable source of TB mortality data in that state. In fact, combined mortality data from the cities of New York, Philadelphia, and Boston from 1871 to 1912 was utilized to describe a decrease in mortality from TB at a meeting of the National Association for the Study and Prevention of Tuberculosis in 1912 [25]. Michigan became the first state to mandate the reporting of tuberculosis in 1888 [26]. New York City was another pioneer in the early efforts to prevent TB; registration of cases was among the activities undertaken beginning in 1893 [27,28].
By the early twentieth century, a national death registry maintained by the U.S. Government Office of Vital Statistics was in place and included mortality data from 10 states and the District of Columbia. At that time, those 11 reporting areas represented approximately 40% of the total U.S. population [29]. The campaign against TB was becoming much better organized by 1904 when the National Tuberculosis Association (NTA) was formed. NTA was an early leader in efforts to decrease TB nationwide through education and advocacy. The NTA leadership of the national association was instrumental in bringing the 6th International Tuberculosis Conference to Washington, D.C., in 1908 and in the formation of state TB associations in Colorado, Kansas, Michigan, Wisconsin, Texas, and West Virginia [30].
Beginning in 1930 nationwide statistics from 48 states on the total number of tuberculosis cases were collected and reported to the national TB program of the U.S. Public Health Service (USPHS). Because of differences in case definitions or uniformity in reporting practices and the lack of complete reporting from all cities and states, interpretation of these surveillance data was difficult. As a result, surveillance data from the years 1930-1951 fluctuated from year to year [31].
In May 1951, for the first time in the United States, reporting standards of tuberculosis morbidity data were defined. In 1952, the first summary report of the Tuberculosis Program, Division of Special Health Services, USPHS was published. The second report published in 1953 was the first report that included reporting from all states, and it is commonly considered to be the first national report of TB cases utilizing a standardized format. All data were reported in aggregate form directly from the states to the National TB Control Program of the USPHS in the Annual Tuberculosis Report [32,33].
The first case definition included a designation of Group A, “active and probably active” cases, and Group B, “other reportable cases.” Group A cases had bacteriological proof of TB or, in those without laboratory evidence, had other significant evidence of disease (e.g., characteristic chest radiograph or clinically active extrapulmonary TB). Group B included previously unreported tuberculosis cases such as those with a history of active disease or previous treatment within the past 5 years. Cases reported as Group B one year were not precluded from being reported as Group A cases in subsequent years. In 1953, 113,531 tuberculosis cases were reported in the United States with 88,919 (78%) classified as Group A [32].
In 1960, the National TB Control Program was transferred from the Public Health Service to the Communicable Disease Center, the precursor to CDC. In 1961, the National TB Control Program revised its recommendations on the reporting of tuberculosis cases. A recommendation that only active cases be counted to assess the incidence of tuberculosis was adopted by some states and became more widely used in 1962. It was also the first year that data reporting U.S. cases broken down by sex and age group became available. Not all new recommendations were applied uniformly by the states. A more complete reporting of cases based on the new recommendations was observed in 1963 [34].
From 1965 to 1980, TB skin test results for household and nonhousehold contacts of active cases were reported for a subset of states. By 1980, that number was 30 states and 25 cities of greater than 250,000 population [35]. A change in case counting criteria that began in 1975 rendered previously published surveillance data not directly comparable to subsequently published data. From 1975 on, cases formerly classified as reactivation TB were included in morbidity data. Additionally in 1975, detailed demographic and epidemiologic information on extrapulmonary cases was published [36].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


