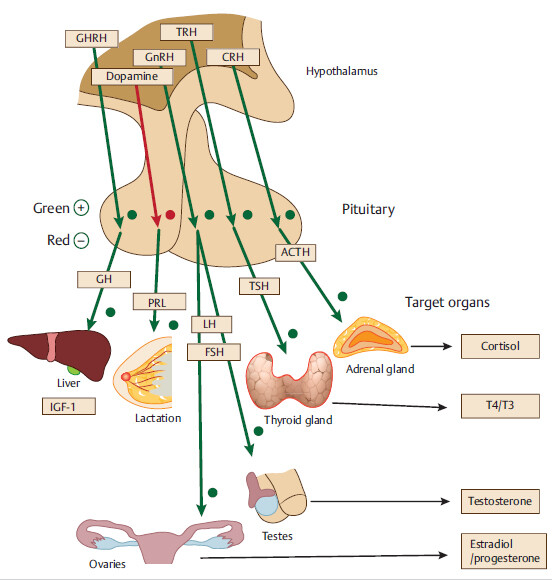
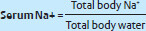2.1 Disorders of the Anterior Pituitary Gland (Adenohypophysis)
Abbreviations: GHRH, growth hormone releasing hormone; GH, growth hormone; IGF-1, insulinlike growth factor-1; Prl, prolactin; ACTH, adrenocorticotropic hormone; FSH, follicle-stimulating hormone; GH, growth hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; TSH, thyroid-stimulating hormone.
2.1.1 Common Features of All Pituitary Adenomas
As pituitary gland is near to the optic chiasm, pituitary adenomas cause compressive visual field defects, which primarily involve the temporal fields causing bitemporal hemianopsia
1 Think of a person always hitting the car on the side, but not on the front or the back of the car.
Large adenoma can compress surrounding pituitary tissue, causing pressure atrophy and hypopituitarism. This leads to sequential loss of anterior pituitary hormones. Growth hormone (GH) and gonadotrophin-releasing hormones (GnRHs) are affected earlier in the disease course, followed by thyroid-stimulating hormone (TSH) and adrenocorticotropic hormone (ACTH).
• Children commonly present with stunted growth due to early development of GH deficiency.
In adults, deficiency of GnRHs (luteinizing hormone [LH] and follicle-stimulating hormone [FSH] deficiency) is more problematic. Men commonly present with impotence and women with amenorrhea.
An adenoma can be functioning (secreting a hormone in excess, as above) or nonfunctioning.
A large functioning pituitary adenoma can present with signs of excess of one of the pituitary hormones and deficiency of other pituitary hormones. For example, a large somatotroph adenoma can present with acromegaly and central hypothyroidism/ hypocortisolemia.
2.1.2 Prolactinoma (Lactotroph Adenoma)
Background: Benign tumor that autonomously secretes prolactin hormone. It is the most common tumor of the pituitary gland.
Pathophysiology/presentation: Excessive secretion of prolactin stimulates mammary glands and also decreases GnRH secretion, causing hypogonadism (decreased LH/FSH production) and the following clinical effects
Presentation scenarios
CCS: 40-year-old female presents with milky breast discharge and oligomenorrhea.
CCS: 55-year-old male presents with decreased libido and impotence. Physical exam reveals visual defects primarily involving bitemporal visual fields.
Work-up: First SIM is to check serum prolactin level and rule out the following conditions that can increase prolactin secretion.
If the above conditions are ruled out and serum prolactin level is high, NSIDx is MRI of the pituitary gland.
2 If a mass is seen in the pituitary area, also evaluate for hypersecretion of other pituitary hormones.
Treatment
Treatment is indicated in any degree of hypogonadism (e.g., amenorrhea) or in patients with bothersome galactorrhea.
3 In postmenopausal women with no visual defects, regular monitoring with MRI and prolactin levels may be done.
Treatment of choice is pharmacologic. Use cabergoline, which has fewer side effects than bromocriptine. (Dopamine agonist decrease prolactin hormone secretion and shrink tumor size.)
Surgical treatment (removal of adenoma) is done when patients fail to respond to medical management. Note that even in patients with significant neurologic symptoms, medical treatment should be tried first.
Radiotherapy is considered when medical and surgical treatments fail.
2.1.3 Acromegaly and Gigantism
Etiology: Excess GH (Growth Hormone) secretion occurs most commonly from a tumor in the pituitary gland. Other rare causes are ectopic GH or GH-releasing hormone secretion from cancers.
Presentation
Workup
First SIDx: measure insulin-like growth factor-1 (IGF-1) level aka somatomedin. Somatomedin (IGF-1) is a substance secreted by liver in response to GH. This is a great surrogate marker for GH level.
If IGF-1 level is elevated, NSIDx is glucose suppression test. GH levels are measured before and after patient ingests 100 g of glucose. Normally, glucose load should suppress GH secretion. Nonsuppressible GH level confirms diagnosis of autonomous GH secretion.
Treatment
Treatment of choice is surgical (transsphenoidal resection of pituitary adenoma).
Medical treatment is indicated in patients who are at high risk for surgery or when surgical therapy fails. Use somatostatin analogues (octreotide or lanreotide) +/- cabergoline, a dopamine agonist (both inhibit GH release). If still not controlled, use pegvisomant—a somatomedin antagonist that blocks somatomedin (GH) receptors.
Radiotherapy is considered when medical and surgical treatments fail.
Management steps for various pituitary adenomas
Cushing’s disease will be discussed in adrenal section of this chapter.
2.1.4 Hypopituitarism
Pathophysiology: Various pathologic processes can involve the pituitary gland and cause hypopituitarism—autoimmune, infectious, trauma, tumor compression, metastasis, infiltrative (sarcoidosis and hemochromatosis), postsurgical removal of pituitary adenoma, etc. Additional causative scenarios include the following.
| ||
Serum cortisol, ACTH, and cosyntropinb stimulation test | ||
a Insulin-induced hypoglycemia was used as a provocative method in the past, but it can be a very uncomfortable experience for the patient, so it is no longer used. | ||
Abbreviations: ACTH, adrenocorticotropic hormone; FSH, follicle-stimulating hormone; GH, growth hormone; GnRH, gonadotropin-releasing hormone; IGF-1, insulin–like growth factor-1; LH, luteinizing hormone; TSH, thyroid-stimulating hormone. | ||
2.1.5 Pituitary Apoplexy
Background: Patients with large pituitary adenoma can develop pituitary apoplexy, an acute clinical syndrome caused by either hemorrhage and/or infarction of the pituitary gland.
Presentation: Hemorrhagic form usually presents with sudden severe headache (“thunderclap” headache) +/- signs of meningism. Look for rapidly worsening visual deficits (due to pressure on optic chiasm).
This may be followed by symptoms due to essential hormone deficiency. Predominant feature is hypotension due to acute adrenal insufficiency.
Management: It is a medical emergency. NSIM is IV fluids and IV hydrocortisone.
2.1.6 Posterior Pituitary Lobe (aka Neurohypophysis)
Posterior pituitary secretes antidiuretic hormone (ADH aka vasopressin) and oxytocin.
5 Oxytocin hormone is discussed further in Obstetrics chapter. ADH will be discussed in this chapter.
ADH promotes reabsorption of free water from the kidneys and maintains serum sodium concentration and plasma osmolarity.
Increased ADH secretion leads to increased total body water resulting in decreased serum Na+ concentration.
2.2 Disorders of Sodium Hemostasis and Antidiuretic Hormone of Posterior Pituitary Gland
2.2.1 Diabetes Insipidus
Pathophysiology: Diabetes insipidus (DI) is either due to deficiency of ADH or resistance to ADH effect. This results in excretion of excessive amount of diluted urine by kidneys, resulting in loss of free water. The resulting polyuria is usually accompanied with compensatory polydipsia.
Presentation: Usually patients with polyuria have intact thirst mechanism, so if they lose 20 L of water, they will drink 20 L of water continuously replenishing the free water loss. In situations where thirst mechanism is impaired (sedatives, altered mental state) or if there is a problem with access to water (long car ride, incarceration), patients can become acutely ill, dehydrated, and may develop acute hypernatremia.
8 Clinical features of hypo/ and hypernatremia are somewhat similar: headache, lethargy, coma, and eventually seizures can develop.
Differential diagnosis of diabetes insipidus
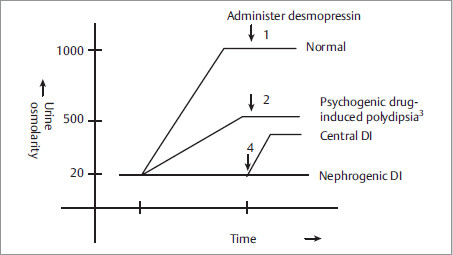
Work-up: NSIDx is serial measurements of urinary and plasma osmolarity (after water deprivation, followed by ADH administration) as shown below.
Fig. 2.3
1,2 Exogenous ADH will not have effect, as ADH is already working at maximum after water deprivation.
3 In psychogenic or drug-induced polydipsia the concentrating ability of kidneys gets impaired. It may take days, after normalizing water intake, for the concentrating ability of kidneys to come back to normal.
4 To differentiate nephrogenic DI from central DI, administer desmopressin (an ADH analogue). More than 50% increase in urine osmolarity after desmopressin indicates ADH deficiency (central DI).
Table representation of the graph above
Management of central/nephrogenic DI:
First step in treatment is to decrease solute intake (low salt and protein diet). This decreases polyuria. (In DI, the kidneys lose the ability to concentrate urine. With fixed urine osmolarity, urine output is primarily determined by amount of solute intake and solute content in urine.)
Second step is to use hydrochlorothiazide (HCTZ) in patients with persistent polyuria. (Diuretics induce a state of partial volume depletion, which increases proximal sodium and water absorption, leading to less delivery of water to distal ADH-sensitive areas.)
Desmopressin is used for central DI, if decreased solute/protein intake and/or HCTZ treatment fails to control polyuria, or if symptoms are severe.
Nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., indomethacin), which increase renal concentrating ability, can also be used as an adjunct.
2.2.2 Hypernatremia
Etiology
Free water loss due to diabetes insipidus.
Hypotonic fluid loss without water replacement
9 Absence of water replacement typically occurs in patients with advanced age, dementia, and mental retardation, because these patients have decreased or diminished thirst response mechanism.
Fluid loss from skin, due to burns or excessive sweating (fever, vigorous exercise, hot weather, etc.).
Fluid loss from gastrointestinal tract, due to osmotic diarrhea (e.g., lactulose ingestion) and diarrhea in infants. (Note: In adults, diarrheal fluid is isotonic.)
Fluid loss from kidneys, due to osmotic diuresis (e.g., diabetic ketoacidosis [DKA], hyperosmolar nonketotic coma [HONK], or drugs such as mannitol).
Management
First step is to look for coexistent volume depletion.
If blood pressure is low (systolic BP ≤ 90 mmHg) and/or heart rate is high (> 90-100 beats per minute), 1st SIM is IV normal saline.
If euvolemic, use IV hypotonic fluids such as 5% dextrose solution in water or if coexistent mild volume depletion is present, use 5% dextrose half normal saline.
The rate of correction of chronic hypernatremia should not exceed > 10 to 12 mEq/L per day, otherwise cerebral edema may develop. For acute hypernatremia of less than 48 hours’ duration, more aggressive correction can be done (1-2 mEq/L correction per hour).
2.2.3 Hyponatremia
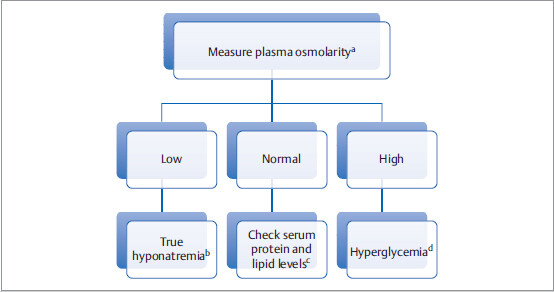
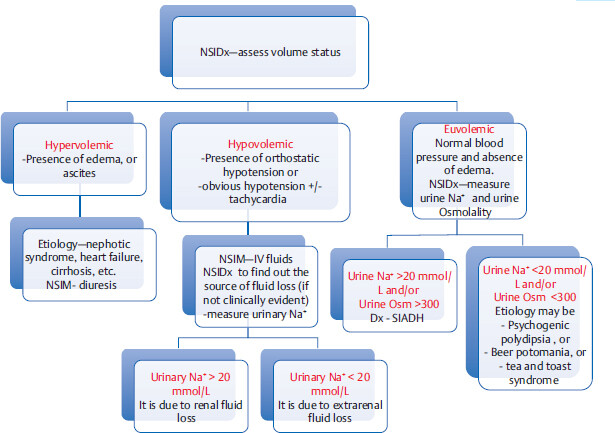
First step is to find out if serum sodium is truly low: Is it true or is it pseudohyponatremia?
aDo direct lab measurement of osmolarity, which might be different from calculated plasma osmolarity.
bIn true hyponatremia, measured plasma osmolarity should be low.
cHypertriglyceridemia and hyperproteinemia can cause a decrease in measured serum Na+ level (pseudohyponatremia) with no change in directly measured plasma osmolarity. This is an artefactual issue.
dHigh serum glucose level decreases measured serum Na+ level (pseudohyponatremia) and increases plasma osmolarity.
11In hyperglycemia, for every 100 mg/dL rise in blood glucose above 100 mg/ dL, measured serum Na+ decreases by 1.6 mEq/L.
For example, electrolyte panel shows glucose level of 804 mg/dL and serum Na+ of 125 mEq/L. Corrected true serum Na+ = 7 × 1.6 + 125 = 136.2 mEq/L.
Explanation: 7 is because glucose is elevated 700 mg/dL above 100 mg/dL in this case. Note: This situation is very common in DKA or HONK patients.
Hypervolemic Hyponatremia
In these conditions, development of hyponatremia is a poor prognostic sign. Level of hyponatremia parallels the severity of the underlying condition. Treatment includes restriction of sodium/ water intake and diuretics.
Hypovolemic Hyponatremia
Measure urine Na+ level to differentiate renal fluid loss from extrarenal fluid loss (if source of fluid loss is not clinically evident).
Euvolemic hyponatremia
Management of true hyponatremia
NSIM along with free water restriction and treatment of underlying cause ↓ | |
121 to < 130 mEq/L with mild symptoms (mild confusion, fatigue, gait disturbance, nausea, vomiting, etc.) | Directed toward underlying etiology: |
≤ 120 mEq/L and/or severe symptoms (e.g., severe confusion, coma, seizures, respiratory arrest) | Hypertonic salineb +/- desmopressin to prevent rapid correction of sodiurmc |
aVasopressin receptor antagonist (tolvaptan, conivaptan, mozavaptan, etc.) can be used adjunctively in SIADH and hypervolemic states. | |
bHypertonic saline is also indicated in acute symptomatic hyponatremia that develops within 24 hours (even if symptoms are only mild). | |
cRate of sodium correction should not be faster than 0.5-1 mEq/L per hour. Rapid overcorrection may result in central pontine myelinolysis (osmotic demyelination), which usually presents with focal neurological deficits like the ones seen in pontine hemorrhage or infarction (severe cases might present with quadriplegic locked-in-syndrome). (See Chapter 10, Neurology for further details). | |
2.2.4 Syndrome of Inappropriate Secretion of ADH
Etiology: SIADH can be caused by multiple processes, such as trauma, infection, malignancy (especially in lung or brain, e.g., lung injury, head injury, pneumonia, encephalitis, small cell cancer) and medications.MRS-1 Important association to remember is small-cell lung cancer.
Pathophysiology Excessive ADH secretion leads to excessive free water reabsorption and hyponatremia.
Treatment: Its treatment is directed toward underlying condition and also depends upon the severity of hyponatremia and symptoms (see table for management of true hyponatremia). Demeclocycline (ADH receptor blocker) is not commonly used nowadays because of increased risk of nephrotoxicity and high cost.
MRS-1 Drugs that can stimulate secretion of ADH (Tip: most of the drugs start with “C”)
Antidepressants SSRIs (Citalopram, sertraline, etc.), Tricyclic antidepressants
2.3 Disorders of Calcium Hemostasis and Parathyroid Gland
2.3.1 Basics of Calcium (Ca2+) Metabolism
Total serum calcium = bound calcium + free calcium.
Bound form: Calcium is a positively charged ion which binds to albumin and other negatively charged compounds (e.g., citrate–, PO4 –, SO4 –).
Free form is the one that is biologically active and closely regulated by parathyroid hormone (PTH).
The two hormones that regulate calcium are PTH and Vitamin D.
If calcium and phosphorus are in opposite directions, think PTH related and if they are both in same direction, think vitamin D related. | |
Patients with hypoalbuminemia (due to liver cirrhosis, protein-losing enteropathy, malnutrition, etc.) can have the following issue with calcium mechanics:
– Decreased total calcium but normal free Ca2+ level. These patients are asymptomatic.
Standard chem-7 or basal metabolic profile (BMP) tests measure total calcium. Hence if serum albumin is low, the calcium needs to be corrected with the help of a formula using serum albumin. We don’t need to know this formula for boards.
A case of masked hypercalcemia:
I had once taken care of a patient, who didn’t have a primary care doctor, who presented with fatigue. Complete metabolic profile revealed high normal calcium level and very low serum albumin. Corrected calcium value was found to be more than 14. She ended up having a diagnosis of severe acute hypercalcemia due to parathyroid adenoma. Hypoalbuminemia was probably a result of severe malnutrition due to hypercalcemia associated gastrointestinal symptoms.
2.3.2 Disorders of Calcium Metabolism
For the tables below, cover the diagnosis column (right most column) and try to explain mechanism of calcium disorder. Again, let us reiterate an important point—If calcium and phosphorus are in opposite directions, think PTH related; if they are both in same direction, think vitamin D related.
Hypercalcemia
↑ /or high Na | N or lowb | NSIDx is 24-hour urinary calcium. | ||
N or lowb | PTH-related peptide (PTHrP) secreted from cancer cells (squamous cell cancer of lung, breast cancer, etc.). This peptide works just like PTHc. | |||
Consider causes that increase bone resorption—prolonged immobilityd, multiple myeloma, hyperthyroidism. (Look for elevated alkaline phosphatase) | ||||
aIn hypercalcemia, PTH should be low if feedback mechanism is intact. | ||||
c Hypercalcemia of malignancy can be due to PTHrP secretion (typically by squamous cell carcinoma) or due to cytokines secreted by osteoclastic metastatic cells (multiple myeloma is a classic example). Cancers at times can cause both types (e.g. breast cancers). | ||||
dPatients who have high bone turnover rate (e.g., Paget’s disease or in children), immobilization can lead to development of significant hypercalcemia (Hypercalcemia of Immobilization). | ||||
Hypocalcemic disorders
Primary hypoparathyroidism (Etiology: DiGeorge syndrome, thyroidectomy complication, parathyroidectomy, polyglandular autoimmune syndrome, etc.) | ||||
↑ e | Chronic renal failurea | |||
↑e | ???b | |||
↑ e | Vitamin D deficiency (osteomalacia in adults and ricketsc in children) | |||
↑ e | Vitamin D resistant ricketsd | |||
aIn renal failure, there are two primary mechanisms: (1) retention of PO4 2 (which chelates calcium), (2) decrease in vitamin D production. | ||||
bLooks like it is a PTH-related disorder with calcium and phosphorus in opposite direction. Calcium is low even though PTH is high. So, PTH must not be working at all. Dx is pseudohypoparathyroidism, where there is a defect in PTH receptor, so even if PTH is present, it does not exert its effect. | ||||
cFor rickets, look for frontal bossing, bowed legs, and enlargement of costochondral junction. | ||||
dPrimary pathology is defect in vitamin D receptors, so even though vitamin D level is normal or high there is lack of effect. | ||||
e ↑ = secondary hyperparathyroidism (Elevated alkaline phosphatase is present in all cases except in pseudohypoparathyroidism.) | ||||
A pediatric patient presents with frontal bossing, bowed legs, and enlargement of costochondral junction. | Hereditary hypophosphatemic ricketsa | ||||
aHeritable disorder in which the primary problem is significant phoshaturia (phosphate wasting). | |||||
Hypercalcemia Clinical features
Gastrointestinal: constipation, abdominal pain, pancreatitis, etc.
Neurological symptoms depending upon severity: difficulty concentration → fatigue, weakness → altered mental status → seizures, coma.
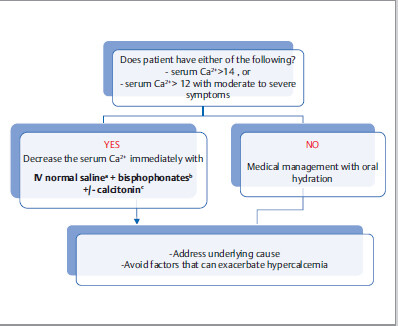
Management
aFurosemide (Lasix) is not routinely indicated in management of hypercalcemia. If patients develop volume overload with IV normal saline, it can be judiciously used.
b Bisphosphonates decrease osteoclastic activity. As opposed to calcitonin, it takes at least few days for the hypocalcemic effect to kick in, but it is more sustained. It is contraindicated in severe renal failure.
Bisphosphonates are also indicated in patients with symptoms related to bone resorption, for example, patient with acute back pain due to metastatic osteolytic lesion.
Denosumab is an alternative treatment of persistent hypercalcemia of malignancy refractory to bisphosphonates.
cIV calcitonin is mostly recommended in patients with serum Ca2+ > 14. It decreases calcium levels faster than bisphosphonates do, but its efficacy is reduced after 48 hours. In severely hypercalcemic patients, hemodialysis can be done as a treatment of last resort.
Primary Hyperparathyroidism
Etiology: MCC is parathyroid adenoma. Other causes include parathyroid hyperplasia/ malignancy.
Workup: After detecting elevated calcium levels, NSIDx is to check serum PTH level.
If elevated or upper side of normal range, NSIDx is 24-hour urinary calcium measurement. If 24-hour urinary calcium is elevated the dx is primary hyperparathyroidism.
16 If 24-hour urinary calcium is low, the dx is Familial hypocalciuric hypercalcemia: High calcium, via calcium-sensing receptors should generally decrease PTH secretion from parathyroid glands and increase calcium excretion from kidneys.
In this rare disorder, calcium-sensing receptors in the parathyroid gland and kidneys are defective. These patients have high normal or high PTH level and low 24-hour urinary calcium excretion, despite high serum calcium. Genetic testing is confirmatory.
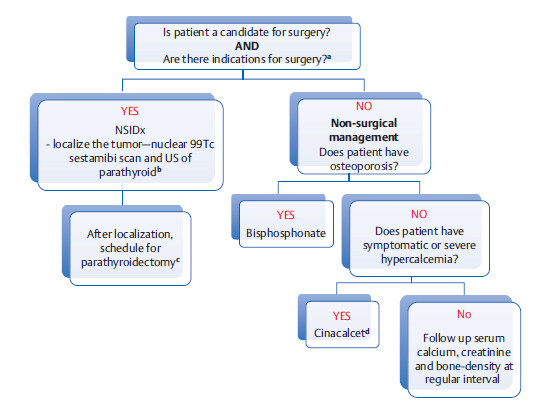
Management of Primary Hyperparathyroidism
a Symptomatic hypercalcemia is an obvious indication. In asymptomatic patients, the following are indications for surgery:
bOther options are CT or MRI scan of parathyroid gland. Localization of tumor is not necessary in patients undergoing nonsurgical management.
cA potential complication of removal of parathyroid adenoma is hungry bone syndrome. (7 to 10 days after surgery with precipitous decline in PTH levels, bones can go into a rapid remineralization phase with increased uptake of calcium and phosphates, resulting in serum hypocalcemia and hypophosphatemia. This is usually self-remitting and transient.)
dCinacalcet decreases PTH secretion by activating calcium-sensing receptors in parathyroid glands (it acts by “mimicking” calcium).
Hypocalcemia Additional etiologies of hypocalcemia
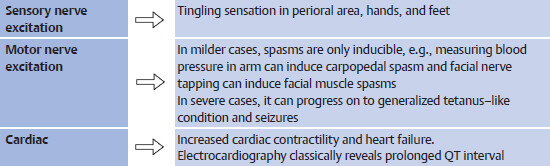
Presentation: Acute hypocalcemia leads to generalized neuromuscular and cardiac hyperexcitability.
Management: Treatment of acute hypocalcemia is with IV calcium gluconate or calcium chloride. Maintenance is with oral calcium.
2.4 Thyroid Hormone
Thyroid hormone controls the basal metabolic rate and Na+–K+–ATPase pump in all our cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree