Use of Noninvasive Methods to Diagnose Cardiovascular Disease in Dyslipidemic Patients
Ilan Gottlieb
João A.C. Lima
The effect of higher levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) on the development of cardiovascular disease (CVD) has been established since the early days of atherosclerosis research and has paid enormous dividends in terms of improving cardiovascular (CV) morbidity and mortality. Statins, more specifically, have been tested in thousands of patients in a myriad of clinical scenarios and have accumulated enough evidence to support their use as one of the most prescribed drug families worldwide.
Nonetheless, dyslipidemia is only one of several risk factors for CVD (it is estimated that more than 200 risk factors have been reported to be associated with CVD), which explains only part of the CV events seen in the general population. The existence of multiple risk factors for CVD occurs due to the highly heterogeneous pathophysiology of atherosclerosis, with multiple genetic and environmental risk factors still poorly understood, which hinders the development of precise probabilistic clinical risk stratification models and probably also a “one size fits all” treatment plan.
Although effective risk-lowering therapies exist, myocardial infarction (MI) or sudden unexpected death remains a very common first manifestation of coronary atherosclerosis. These attacks often occur in patients who are not receiving the benefits of preventive therapies of proven efficacy because their arterial disease was unrecognized (asymptomatic) and/or they had been misclassified by conventional risk factors and assigned a treatment goal at odds with their actual burden of atherosclerosis. In one study, 75% of the patients previously without known CVD admitted to the hospital for their first MI did not fulfill guidelines criteria to receive lipid-lowering medications immediately before the event (1). These criteria are mostly based on the Framingham risk stratification model and have many caveats (see also Chapter 15). For example, they apply only for persons without known heart disease, the risk algorithm encompasses only coronary disease—not other heart and vascular diseases, and the study population is almost all whites. In addition, the Framingham risk score estimates the risk of developing coronary disease within a 10-year time period. This risk score may not adequately reflect the long-term or lifetime risk of CVD in young adults (one in two for men and one in three for women). The presence of any CVD risk factor requires appropriate attention because a single risk factor may confer a high risk for CVD in the long run, even if the 10-year risk does not appear to be high. Since age is a prominent determinant of the coronary disease risk score, the 10-year hazards are, on average, high in older persons. This may overidentify candidates for aggressive interventions and underidentify younger at-risk patients.
THEORETICAL CONSIDERATIONS
Support for Early Detection of CVD
Atherosclerosis formation starts very early in life and is usually well established by early adulthood (see also Chapter 12). Since early atherosclerosis rarely results in events, it is thought to be a benign condition, delaying treatment until more advanced stages. This clearly is shortsighted. It overlooks the fact that the early lesions are the precursors of the later, clinically threatening lesions. They are not two distinct clinical entities that are handled separately, but the same pathological process that spans over decades and must be dealt as soon as possible. It is illogical to assume that a person with advanced disease would have the same clinical benefits from atherosclerosis treatment strategies as another person with early-stage disease. Our oncology colleagues have recognized this forever.
Due largely to economic reasons, industry has focused research on secondary prevention treatment (i.e., patients with clinically established CVD) in spite of primary prevention. This distortion has encouraged physicians to wait until disease states become symptomatic, when it may be too late for interventions to improve outcomes (see also Chapter 5). One meta-analysis (2) of the four largest randomized clinical trials comparing high-dose statins with standard-dose statins in secondary prevention (pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 (PROVE IT-TIMI 22), aggrastat to zocor (A-to-Z) trial, treating to new targets (TNT) trial, and incremental decrease in end points through aggressive lipid lowering (IDEAL) trial demonstrated that even among patients taking very high statin doses (atorvastatin 80 mg/day or simvastatin 80 mg/day) and having a mean low-density lipoprotein (LDL) of 75 mg/dL, event rate was as high as 29% at studies’ follow-up (let alone lifetime risk).
But does early management result in better outcomes? Recent discoveries may help answer this question. A new gene importantly involved in regulation of the LDL receptor has recently been identified (3). This gene codes for proprotein convertase subtilisin/kexin-type 9 (PCSK9), a protease that decreases the number of LDL receptors expressed in the liver
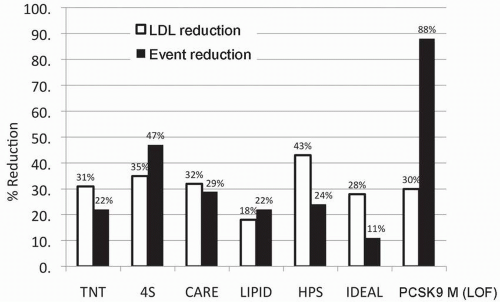
(see also Chapter 8). Gain-of-function mutations in PCSK9 result in an increase in PCSK function, leading to a decrease in LDL receptors and an increase in LDL-C levels in blood similar to the levels of those with defects in the LDL receptor per se (see also Chapter 8). Conversely, loss-of-function mutations in the PCSK9 gene result in a decrease in PCSK9 activity, a higher number of LDL receptors, increased clearance of LDL-C from blood, and lower LDL-C levels. While statin trials lower LDL-C and the CVD event rate by about 30%, carriers of the gain-of-function PCSK9 mutations also have an LDL-C that is 30% lower than controls, but the CVD event risk, on the other hand, was reduced by 88% (4). Figure 6.1 demonstrates the percent reductions in LDL-C and event rates seen in large clinical statin trials, compared with those seen in the people carrying the mutant PCSK9 gene. The implication is that having a low LDL-C starting at birth almost triples the magnitude of the effect on CVD risk compared with the risk reduction found in a 5-year trial of those with established coronary disease, a strong argument for earlier treatment of elevated LDL-C.
(see also Chapter 8). Gain-of-function mutations in PCSK9 result in an increase in PCSK function, leading to a decrease in LDL receptors and an increase in LDL-C levels in blood similar to the levels of those with defects in the LDL receptor per se (see also Chapter 8). Conversely, loss-of-function mutations in the PCSK9 gene result in a decrease in PCSK9 activity, a higher number of LDL receptors, increased clearance of LDL-C from blood, and lower LDL-C levels. While statin trials lower LDL-C and the CVD event rate by about 30%, carriers of the gain-of-function PCSK9 mutations also have an LDL-C that is 30% lower than controls, but the CVD event risk, on the other hand, was reduced by 88% (4). Figure 6.1 demonstrates the percent reductions in LDL-C and event rates seen in large clinical statin trials, compared with those seen in the people carrying the mutant PCSK9 gene. The implication is that having a low LDL-C starting at birth almost triples the magnitude of the effect on CVD risk compared with the risk reduction found in a 5-year trial of those with established coronary disease, a strong argument for earlier treatment of elevated LDL-C.
Therefore, early detection of atherosclerosis itself before symptoms occur can provide a major opportunity to prevent many CVD events. Because screening to identify subclinical or asymptomatic atherosclerosis could confer great public health benefit, it is surprising that it has not yet been incorporated into national and international clinical guidelines.
Imaging Atherosclerosis Burden
Carotid intima-media thickness (CIMT) and coronary calcium score (CCS) are two modalities that provide an overall estimate of atherosclerosis burden that have been shown in large clinical trials to predict CVD events. While CIMT assumes that being a systemic disease atherosclerosis can be measured interchangeably between carotids and coronaries, CCS assumes that the amount of coronary calcification (which is very specific for atherosclerosis) is a good estimate of the total plaque burden in the coronary arterial system.
The potential to quantify the burden of atherosclerosis by nonenhanced computed tomography (CT) attracted the attention of cardiologists, radiologists, and epidemiologists early in the development of electron-beam CT (5). Both the power and main limitations of this approach to predict risk and quantify disease progression can be anticipated from current knowledge of the pathogenesis and epidemiology of atherosclerosis in industrialized populations. Coronary calcification as assessed by CT reflects only the calcified components of coronary atherosclerotic plaques involving epicardial coronary arteries. It is therefore proportional to total epicardial plaque burden (6) but does not reflect microvascular disease (7).
Coronary Calcification and CVD Risk Factors
The magnitude of coronary calcification predicts the development of clinical events and is also related to most of the known etiologic factors that determine atherosclerosis such as TC, LDL-C, blood pressure, cigarette smoking, and family history of CVD (8,9,10,11). Coronary calcification adds to the prediction of coronary events over and above the Framingham risk score (10,12,13) and outperforms C-reactive protein (14) and CIMT (15). Greenland et al. (12) have demonstrated that asymptomatic individuals with intermediate risk according to the Framingham criteria but with calcium scores >300 had an annual hard event rate of 2.8% and would therefore be classified as high risk for CVD events. In that study, the best estimates of relative risk demonstrated that a calcium score >300 conferred a hazard ratio of approximately 4 compared with a calcium score of 0. This implies that the estimated risk in a Framingham intermediate-risk patient with a calcium score of 0 would be reduced twofold, while the corresponding risk for a similar Framingham intermediate-risk patient with a score >300 would be increased twofold, which would lead to reclassifying the latter patient into a high-risk group. On the other hand, while
the evidence from prospective studies demonstrating the predictive power of coronary calcification to detect CVD events in asymptomatic individuals is compelling, there is no direct evidence that such screening will lead to reduced morbidity and mortality caused by CVD.
the evidence from prospective studies demonstrating the predictive power of coronary calcification to detect CVD events in asymptomatic individuals is compelling, there is no direct evidence that such screening will lead to reduced morbidity and mortality caused by CVD.
CT and CCS measurements entail relatively low risks; the radiation exposure is limited to 1.0 to 2.0 mSv, and the test does not require the use of iodinated contrast agents. Conversely, the main intrinsic limitations of coronary calcification as a predictor of CVD events relate to both the very high prevalence of coronary atherosclerosis in industrialized societies, as well as the fact that coronary calcification represents only one of the components of atherosclerotic plaques that may develop late in the natural history of a single plaque (see also Chapter 3) (16,17). Therefore, the amount of calcium accumulated in any given coronary arterial segment reflects not only the magnitude of plaque burden, but also the period of time during which plaques were exposed to the factors that underlie calcification. While the potential contributions of inflammatory, hormonal, metabolic, and physical factors believed to underlie coronary calcification are still incompletely understood, the process is believed to represent a natural biologic response to arterial wall injury, activated for the purposes of increasing arterial wall stiffness and theoretically render plaques less vulnerable to rupture or undue deformation caused by mechanical and biologic stresses.
This traditional viewpoint has, however, been challenged by empiric observations suggesting that at least initially, plaque calcification could increase as opposed to reduce the risk of plaque rupture by creating high-stress interfaces with other less stiff plaque components (18,19). Given the preceding considerations, therefore, the risk associated with coronary calcification for any given individual is considered to be best represented when age, gender, and risk factors are taken into consideration. For example, a calcium score of 17 in a patient with strong family history of premature sudden death caused by atherosclerosis has a completely different meaning if the patient is 27 as opposed to 67 years old.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree