Surveillance for Zoonotic Diseases
Centers for Disease Control and Prevention, Atlanta, GA, USA
Introduction
Zoonotic diseases are infections transmitted between animals and humans and include diseases such as rabies, avian influenza, plague, salmonellosis, anthrax, ringworm, tapeworm infection, and scabies [1]. A recent survey identified more than 1,400 species of human disease–causing agents, over half (58%) of which were zoonotic [2]. Moreover, nearly three-quarters (73%) of infectious diseases considered to be emerging or reemerging were zoonotic [2]. Zoonotic disease–causing agents pose continuing threats to human and animal health, can cause devastating economic impacts, and can be modified for use as biological weapons.
Transmission
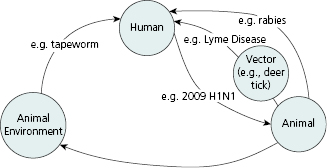
As illustrated in Figure 10.1, zoonotic infections can be transmitted from animals to humans directly through contact with an infected animal (e.g., rabies), indirectly through consumption of drinking water or food contaminated by an infected animal (e.g., salmonellosis) [3], or through contact with the animal’s environment (e.g., fecal contamination in animal exhibits) [4,5]. Routes of exposure can include inhalational routes (e.g., anthrax), fecal-oral routes (e.g., tapeworm,), transfer from contaminated fomites (e.g., influenza), or dermal routes (e.g., cutaneous larva migrans). Some zoonotic diseases can also be transmitted between animals and humans through vectors such as fleas (e.g., plague), deer ticks (e.g., Lyme disease) and mosquitoes (e.g., West Nile virus) [6]. Finally, humans can transmit diseases to animals (reverse zoonosis) [3], as occurred during the 2009 H1N1 influenza pandemic when several species of animals, including pigs and cats, were likely infected with potentially fatal respiratory infections by their owners [7,8].
Public Health Risk
Although all humans are at some risk for exposure to zoonotic infections, some populations, such as children, animal care workers and individuals with chronic diseases, are at higher risk. Infections in children tend to result from a lack of understanding of how diseases are transmitted, frequent hand-to-mouth contact, and improper hand washing [9,10]. Children frequently attend petting zoos and other animal venues and can be exposed to gastrointestinal pathogens such as Escherichia coli O157:H7, Salmonella, and Cryptosporidium shed by asymptomatic animals [10]. In addition, children between 1 and 4 years of age are the segment of the population most frequently infected with toxocariasis [11], a parasitic infection transmitted from dogs and cats [12], which can cause visceral larval migrans [13]. Areas such as public parks or playgrounds can be contaminated with Toxocara through feces of free-roaming dogs and cats [13,14], and children playing in these areas can ingest eggs through frequent hand-to-mouth activity [11,12].
Veterinarians are an occupational group that is at increased risk for zoonotic diseases because of routine and direct contact with animals [15]. Other at-risk occupations include agricultural workers [16,17], veterinary staff, pet store employees, animal breeders, and animal distributors [18,19]. Furthermore, multiple cases of laboratory-acquired infections have been reported in individuals working with zoonotic pathogens in clinical laboratories and research facilities. According to the U.S. Department of Agriculture (USDA) National Animal Research Center, brucellosis infections acquired in the laboratory are most commonly reported from inhalational exposure [20–22]. Common laboratory behaviors, such as handling specimens on an open bench and aerosol-generating procedures, place workers at high risk for exposure to Brucella [20].
Immunocompromised individuals, such as persons living with HIV/AIDS, organ-transplant recipients, or pregnant women, are also at increased risk for zoonotic infections and often develop more severe diseases than immune-competent individuals [23]. Bartonella hensalae infections (cat scratch fever) can result from exposure to infected cats and have been reported to cause bacillary angiomatosis, disseminated infection, endocarditis, and death in organ transplant recipients [24]. In addition to increased severity of disease, infection during pregnancy with some zoonotic infections such as toxoplasmosis, listeriosis, or brucellosis can result in fetal infections, pregnancy loss, or congenital defects [25–27].
Emerging Zoonotic Disease and Global Impact
The global movement of humans, animals, and animal products has contributed to the impact of zoonotic diseases in humans [28]. Rapid increases in human populations have led to reductions in wildlife habitats and major ecological changes [29,30].The expanding use of native habitats for housing or agriculture has increased contact between humans and animals, increasing the spread of disease agents [31] In addition, climate change has led to alterations in temperature and rainfall in some areas [30]. These environmental changes allow reservoirs or vectors for diseases such as dengue and Lyme disease to expand to new regions [31–33], resulting in the exposure of naive populations to new zoonotic diseases.
International trade and the ability of people and animals to travel quickly around the world allow for the equally swift spread of zoonotic disease [34]. This can occur through a variety of human activities—both legal and illegal wildlife trade and translocation of animals to new areas; consumption of exotic foods, often from live animal or bushmeat markets; and intensified production of food animals and importation of animal products [29,34,35]. Movement of animal and animal products can introduce novel or emerging zoonotic diseases into naïve human or animal populations, some of which may be competent reservoir hosts for the newly introduced pathogen. Estimates suggest that the wildlife trade results in more than 1 billion direct and indirect contacts among wildlife, humans and domestic animals annually [35]. In many countries there is minimal surveillance for live animal imports or imported wildlife products. Minimal surveillance prevents the identification of wildlife trade–related health risks to the public, agricultural industry, and native wildlife [36] and has led to outbreaks of zoonotic diseases including monkeypox and psittacosis in several countries [37,38].
Illegal wildlife trade may pose an even more significant public health problem. Surveillance of illegally imported animals or animal products is limited [39]. Most illegal wildlife seizures originate in Southeast Asia, a hotspot for emerging zoonotic diseases because of rapid population growth, high population density, and high biodiversity [38,40]. Herpesvirus and Simian foamy virus, both of which infect humans, have been found in samples of bushmeat illegally imported into the United States [36], suggesting that zoonotic disease transmission through illegal movement of wildlife or wildlife products is likely to occur.
Zoonotic disease outbreaks place a tremendous economic burden on affected countries because of trade restrictions, travel warnings or restrictions, public health efforts to contain the disease outbreak, culling of exposed or diseased animals to prevent spread, and loss of confidence in animal products [34]. Over the past 2 decades, economic losses resulting from emerging zoonotic diseases have totaled more than $200 billion [34]. In 2009, the H1N1 influenza pandemic (referred to as “swine flu” in early media reports) resulted in $1.3 billion in lost revenue to the pork industry. Within 1 month after initial detection of the outbreak, 27 countries had placed official or unofficial bans on pork from the United States because of unjustified claims that the virus could be transmitted through consumption of pork [41]. Similarly, annual costs to government and industry for control measures during the bovine spongiform encephalopathy (BSE) outbreak in the United Kingdom were estimated at $858 million [42].
Zoonotic Disease Surveillance
Surveillance is essential for controlling infectious diseases and provides critical information on the spread of disease to new areas or hosts. Surveillance can also be used to inform decisions regarding control measures [43]. Surveillance for zoonotic diseases should utilize a multidisciplinary “One Health” approach which incorporates the complex interactions between human and animal health and the environment, as well as the impact of policy, agriculture, and trade on zoonotic diseases [44].
Under a “One Health” approach, human and animal disease surveillance [43–45] are integrated, because disease surveillance in animals offers opportunities to recognize outbreaks or emergence of new diseases before transmission to or detection in human populations [43,44,46]. Effective surveillance hinges on collaboration and communication between public health, medical, and veterinary partners [44,47]. To increase this collaboration, the American Veterinary Medical Association (AVMA), the American Medical Association (AMA), and Centers for Disease Control and Prevention (CDC) have joined together to promote movement toward an effective, integrated response to public health and animal health emergencies [28].
Approaches to Surveillance
To establish and maintain effective systems, zoonotic disease surveillance relies on multiple surveillance methods in human and animal populations—active, passive, laboratory, syndromic, and sentinel surveillance, as well as biosurveillance.
Active surveillance involves outreach to specific groups to collect data on zoonotic diseases in populations and can detect less common diseases or those targeted for eradication. During the worldwide campaign to eradicate rinderpest, as cases became more infrequent, the Global Rinderpest Eradication Program moved to active surveillance using report registries, questionnaire surveys, participatory epidemiology and investigations, and clinical surveillance for new infections [48]. Active surveillance is also used by CDC’s Active Bacterial Core Surveillance program to detect methicillin-resistant Staphylococcus aureus (MRSA) and by the USDA’s bovine spongiform encephalopathy testing program [49,50]. Unfortunately, active surveillance is often labor- and resource-intensive, so it may not be practical for some zoonotic diseases or settings [51].
Passive surveillance is a less-costly option that relies on voluntary reporting by groups such as physicians, veterinarians, or public health workers. Passive surveillance systems are important components of zoonotic disease surveillance worldwide since they are often easier to implement than active surveillance systems, at least in part because there is less reliance on technology [51] Although requirements vary across public health jurisdictions, high-consequence human infections of animal origin (e.g., brucellosis, hantavirus pulmonary syndrome) are reportable in all jurisdictions and they are nationally notifiable. Data on these and all other nationally notifiable diseases are submitted to a national level surveillance system coordinated by the CDC [52].
Laboratory surveillance, a critical element of a robust zoonotic disease surveillance system, is particularly useful for the detection of rare zoonotic infections in human or animal populations [34,53]. Laboratory-based surveillance is often more timely and better than that of clinical surveillance because of automation of electronic laboratory reporting [54]. Laboratory capacity, however, is often in areas most at risk of zoonotic disease outbreaks [34]. Moreover, in some cases it is hard to detect some pathogens through laboratory methods (e.g., Franciscella tularensis) [55].
Syndromic surveillance identifies unexpected changes in prediagnostic information from a variety of sources to detect potential outbreaks [56]. Sources include work- or school-absenteeism records, pharmacy sales for over-the-counter pharmaceuticals, or emergency room admission data [51]. During the 2009 H1N1 pandemic, syndromic surveillance of emergency room visits for influenza-like illness correlated well with laboratory diagnosed cases of influenza [57]. Since syndromic surveillance relies on prediagnostic information, a case definition that accurately captures early clinical signs and symptoms of a disease is essential to minimize the reporting of unrelated diseases and to increase specificity of the system [51].
Sentinel surveillance focuses on disease detection activities among specific subpopulations. Sentinel populations are often chosen based on attributes that make the disease easier to detect or the subjects more convenient to sample. Likewise, a clear relationship between the sentinel and target population is necessary to extrapolate findings from one population to the other [58]. Sentinel surveillance systems can detect pathogen spread into new areas, changes in prevalence or incidence of a pathogen over time, the rate and direction of pathogen spread, and the efficacy of control interventions [59]. Sentinel populations may serve as early warnings of increased risk to the target population. Examples are the use of coyotes as a sentinel for plague in humans in the western United States [60] and the use of avian, mosquito, and climatic data to model the risk of human West Nile cases [61]. Monitoring of sentinel populations may be more cost-effective than monitoring of the target population because fewer samples may be needed or subjects may reside in a smaller geographic area [58]. Although not currently well utilized, surveillance systems using companion animals as sentinels have the potential to detect a wide array of zoonotic diseases [3] in a sensitive and timely manner.
Biosurveillance is relatively new approach that involves analysis of health-related data for early threat and hazard warnings, early detection of events, and rapid characterization and response to minimize adverse health effects [62]. Although biosurveillance incorporates all hazards (e.g., biological, chemical, radiological), biological agents most likely to be used in a terrorist attack are predominantly zoonotic [63]. Biosurveillance may involve syndromic surveillance of hospital admissions, laboratory surveillance from sources such as the Laboratory Response Network, or monitoring of the ambient air or environment [51]. Regardless of the surveillance strategy used, collection of timely and accurate data to provide situational awareness of population health is a primary goal [62].
Integrated Approach to Surveillance in Humans and Animals
Barriers to integrated animal and human surveillance in the United States include technology issues and concerns with data sharing and trust among stakeholders [43]. Only 19% of surveillance systems collect both human and animal data for emerging zoonotic diseases [44] and, only half of zoonotic disease data collected from animals or humans was electronically analyzed [64]. This causes a communication barrier between animal and human health officials and slows the response to human disease outbreaks [34]. However, there are some U.S. programs that have successfully integrated animal and human surveillance. The National Antimicrobial Resistance Monitoring System (NARMS), for example, is a surveillance system that involves collaboration between the U.S. Food and Drug Administration (FDA), USDA, and CDC to monitor for antimicrobial resistant pathogens in retail meats, animals, and humans, respectively. Laboratory surveillance for emerging pathogens may suffer from lack of available testing procedures. In some locations, collection of samples for testing may be unpleasant, arduous or hazardous [43]. Also, clinical signs and symptoms as well as severity of illness often vary among different species infected with the same pathogen [34]. Advances in laboratory techniques for screening and detection of pathogens offer opportunities for enhanced detection of emerging zoonotic diseases and should lead to improvements in surveillance for novel organisms in both human and animal populations [43].
Novel Zoonotic Disease Surveillance Systems
There are free or low-cost sources of data that may provide reasonable alternatives to formal zoonotic disease surveillance systems [65].
Several Internet-based reporting systems have been described. ProMED, which has more than 60,000 subscribers in 185 countries, uses in-country infectious disease experts to validate reports of emerging disease outbreaks and provides a model for an affordable, Web-based surveillance system for resource-poor countries [43,45]. HealthMap, another news media–based surveillance system, allows users to search for specific diseases and create maps to display similar disease outbreaks and patterns of movement [54]. Data from these Internet-based systems are not as verified as the data from traditional reporting structures; however, these new systems offer advantages in terms of scalability, coverage, and timeliness [66] and provide powerful new tools for real-time reporting and communication of surveillance data.
Another potential tool for zoonotic disease surveillance is the use of mobile phone technology. Participatory surveillance, which collects data contributed by individuals or communities to better understand disease transmission, was developed to meet the needs of the Global Rinderpest Eradication Program [67]. The use of mobile phone–based surveillance is particularly appealing since increases in network coverage, portability, and ease of access have made this technology readily available in many remote areas [43,66]. Community-based reporting systems can augment traditional disease reporting and surveillance in remote areas currently underserved by public health infrastructure.
Lastly, an innovative surveillance model of wildlife populations has been developed to detect novel zoonotic pathogens at high risk of spillover to human populations in resource-poor countries. PREDICT (http://avianflu.aed.org/eptprogram/pdf/Predict_pager_June2010.pdf), an active surveillance model developed by the Emerging Pandemic Threats Program of the U.S. Agency for International Development (USAID), uses risk modelling and sample collection from wildlife in 20 different countries to locate high-risk areas for zoonotic infection where considerable contact between animals and humans occurs [68].
Bioterrorism
The intentional use of zoonotic pathogens as bioterrorism agents became a national concern in the United States in 2001 following the intentional exposure of several individuals to letters contaminated with anthrax delivered through the U.S. Postal System. Most potential bioterrorist agents are zoonotic, can be intentionally introduced into a country to harm human and/or animal health, and may have devastating economic implications [69]. Significant concerns about the threat of agroterrorism (i.e., a bioterrorist attack on agriculture), namely the economic impact on livestock, poultry, and plant production also exist [70].
CDC and the USDA are responsible for monitoring reports of biological agents and toxins and have developed a select agents and toxins list based on the ability of pathogens to cause severe harm to human and animal health. Laboratories are required under federal law to report the identification of a select agent or toxin to CDC or USDA [71,72]. For zoonotic agents, animals can often be used as early warning sentinels of a bioterrorist attack. For example, Q fever may first be detected by a large increase of abortions in animal herds. In some cases, bioterrorism events are first detected by veterinarians who report unusual animal outbreaks to their local health department [69].
Stakeholders
Zoonotic disease surveillance is dependent on collaboration and communication between animal-health and human-health partners, and many national and international organizations are involved. Globally, the World Health Organization (WHO) and the World Organization for Animal Health (Office International des Epizooties, OIE) are involved in zoonotic disease surveillance. The Food and Agricultural Organization of the United Nations (FAO) also participates in zoonotic disease surveillance by monitoring the food supply for livestock diseases that can affect human health [34]. FAO has a joint initiative with WHO and OIE to provide a tripartite global disease tracking system for major animal and zoonotic diseases, called the Global Early Warning and Response System (GLEWS) [73]. In the United States, the USDA has responsibility for animal health surveillance, and CDC is the human health surveillance equivalent at the national level. These two agencies typically collaborate around zoonotic disease issues and outbreaks. During Salmonella outbreaks linked to live-poultry contact, the CDC and the USDA’s National Poultry Improvement Program work together to investigate source poultry flocks and inform and educate hatcheries, agriculture feed stores, and the public on ways to reduce infections in poultry and humans. In addition, the CDC and the FDA’s Center for Veterinary Medicine collaborate on surveillance for zoonotic diseases related to animal feed, feeder rodents, and small turtles. At the state and local levels, health departments collaborate with physicians and healthcare providers, veterinarians, and laboratories to detect unusual health events and investigate outbreaks.
National Surveillance and Reporting
In the United States, states mandate which human and animal diseases are reportable within their borders; and local jurisdictions within states can make additional reporting requirements. The five zoonotic diseases most commonly listed as reportable are brucellosis (50 states), anthrax (50 states), rabies (49 states), exotic Newcastle disease (48 states), and highly pathogenic avian influenza (47 states) [74]. Nationally, the USDA in collaboration with the U.S. Animal Health Association (USAHA) and the American Association of Veterinary Laboratory Diagnosticians (AAVLD), collect data monthly on confirmed OIE-reportable diseases from state veterinarians through the National Animal Health Reporting System (NAHRS) [75]. The CDC and the Council of State and Territorial Epidemiologists (CSTE) collaborate in updating a list of nationally notifiable diseases that includes several zoonotic diseases (Table 10.1).
Table 10.1 Zoonotic diseases reportable to in humans and animals.5
| Human cases | Human and animal cases | USDA (animal cases): |
|---|---|---|
| Arboviral diseases2: | Anthrax1,3,4 | Bovine spongiform encephalopathy2 |
| Babesiosis1 | Brucellosis1,3,4 | Chlamydiosis (avian)2 |
| Botulism1,3 | Glanders3,4 | Camelpox2 |
| Cryptosporidiosis1 | Hendra virus3,4 | Cysticercosis (porcine)2 |
| Ehrlichiosis1 | Melioidosis3,4 | Echinococcosis/hydatidosis2 |
| Giardiasis1 | Nipah virus3,4 | Highly pathogenic avian influenza (HPAI)2,4 |
| Hantavirus pulmonary syndrome1 | Rift Valley fever virus3,4 | Japanese encephalitis2 |
| Listeriosis1 | Venezuelan equine encephalitis virus3,4 | Leishmaniosis2 |
| Lyme disease1 | Newcastle disease virus2,4 | |
| Monkeypox virus3 | Screwworm2
| |
| Novel influenza A virus infections1 |



