Key points
- Women with diabetes have particular health issues and care needs. These include pregnancy and childbirth, Polycystic Ovarian Syndrome (PCOS), and menopause.
- Good control before and during pregnancy reduces the risks to mother and baby.
- Better outcomes are achieved if the pregnancy is planned and normoglycaemia maintained before, during, and after pregnancy.
- Insulin is required in Type 2 diabetes during pregnancy and breastfeeding.
- Existing renal disease and retinopathy may deteriorate during pregnancy.
- PCOS is associated with obesity, insulin resistance, GDM, and infertility.
Rationale
Hormonal imbalance associated with PCOS and menopause affect glucose homeostasis and mental well being. Coordinated care and prepregnancy planning is necessary to ensure optimal outcomes for mother and baby. Planning for an optimal delivery begins in adolescence with diabetes education and sexual counselling.
Diabetes can develop during pregnancy, gestational diabetes. Most non-diabetic women are screened for diabetes during pregnancy so that blood glucose levels can be controlled to avoid the risks that having diabetes places on both mother and baby. Women particularly at risk are those with a history of diabetes in the family, diabetes during a previous pregnancy, or previous delivery of a large baby. There is increasing evidence that maternal hyperglycaemia has a lasting legacy for the child predisposing them to obesity in adolescence and Type 2 diabetes, and possibly memory deficits.
Polycystic Ovarian Syndrome
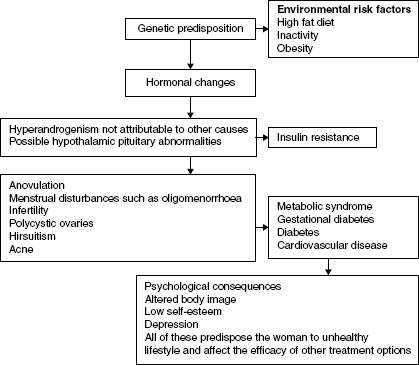
Polycystic Ovarian Syndrome (PCOS) is common, occurring in 6–10% of women in the reproductive age and 28% of obese women. Seventy five percent of women with PCOS are overweight or obese. PCOS has long term implications including menstrual irregularities, infertiliy, gestational diabetes (GDM), impaired glucose tolerance and insulin resistance, Type 2 diabetes, and emotional stress and body image concerns. Hyperinsulinaemia is associated with obesity and stimulates ovarian androgen production, insulin resistance, and cardiovascular disease.
Figure 14.1 Risk factors for Polycystic Ovarian Syndrome and the possible long-term effects. There is still controversy about the diagnostic criteria and several degrees of severity exist. There is no single diagnostic test: diagnosing PCOS is process of exclusion. Diagnostic criteria include National Institute of Health criteria, Rotterdam criteria, and International Androgen Excess Society criteria.
The risk factors for PCOS are shown in Figure 14.1. Making the diagnosis is a process of exclusion and consists of taking a careful family and individual history, hormone levels:
- Testosterone (normal <2.4 mmol/L in women).
- Sex hormone binding globulin (SHBG): likely to be low in PCOS (normal range 30–90) and is often secondary to insulin resistance and results in higher levels of free testosterone.
- Free androgen index (FAI): likely to be high in PCOS (normal range 1–5%).
FAI is more useful than testosterone unless the laboratory can test for free testosterone. Pelvic ultrasound to determine whether the ovaries are polycystic and whether the endometrium is thickened if oligo or amenorrhoea is present may be indicated (Zawazki et al. 1992; Rotterdam Concensus Workshop 2004; Teede et al. 2007). Determining follicle-stimulating hormone (FSH), thyroid stimulating hormone (TSH) and beta human chorionic gonadotrophin (HCG) and prolactin levels may be necessary to exclude other conditions.
Hormone evaluation should be undertaken before prescribing oral contraceptives (OC) because OC can mask the hormonal changes associated with PCOS. Each woman should be individually assessed for cardiometabolic risk before commencing OCs (Yildiz 2008).
A multidiscipline supportive team approach is important to address the various underlying features of PCOS. Reassurance and education about diet and exercise and weight management aiming to lose 5–10% of weight at diagnosis, the need for regular follow up is important. The PCOS Association of Australia provides a useful website. In addition, counselling about fertility may be needed. Because PCOS is associated with insulin resistance and usually obesity, blood glucose and lipids should be checked regularly and an OGTT performed if indicated because fasting glucose is inadequate in PCOS (Teede et al. 2007) (see Chapter 1). Cardiovascular assessment should be undertaken on a regular basis. Advice about diet and exercise are essential and psychological counselling may be required.
The dietary and exercise advice outlined in Chapter 4 is applicable to women with PCOS. Weight loss has been demonstrated in women with PCOS consuming the following diets: Atkins, Ornish, Weight Watchers, Zone, CSIRO Total Wellbeing Diet (Moran et al. 2003). The woman’s food preferences should be considered and the diet should conform to dietary recommendations. If diet and exercise do not control blood glucose, metformin may be added to the diet and exercise regimen. The commencing dose is 500 mg of slow release metformin daily increasing to 2 g per day. Experts recommend giving metformin at night (Teede et al. 2007; Alberti et al. 2007) and there is evidence to support such a recommendation but metfromin is not currently approved for night dosing in Australia.
Metformin helps regulate the menstrual cycle and reduce hirsutism and the progression to diabetes by ∼50 in high risk women (Alberti et al. 2007; Teede 2007). The role of metformin in improving fertility is unclear and it is not first-line fertility treatment. It may be more effective in lean women (De Maria 2007). Nevertheless, fertility should be discussed if metformin is commenced because of the chance of becoming pregnant. Obesity affects fertility independently of PCOS, thus loosing weight is essential if the woman wants to have a child. Age may also be a factor. Fertility declines from ∼28 years and falls dramatically after age 35 and again after age 40.
Oral contraceptives (OC) may be indicated to help reduce hirsuitism and control menstrual irregularities. For example, low dose combined OCs such as Ethinyl Estradiol 20 μg increases SHBG and reduces free androgen levels. At higher doses OCP exacerbate insulin resistance (Meyer et al. 2007). Hair removal products or topical antiandrogens such as Vaniqua may be indicated, and cosmetic electrolysis or laser as a last resort. Obesity exacerbates hirsuitism so managing weight will help normalise androgen levels. The risks and benefits of all these options need to be carefully discussed with the woman considering her individual risk profile.
Antiandrogens such as spironolactone 50 mg BD or cyproterone acetate 25 mg daily for days 1–10 of the active OCP tablets may be prescribed to enhance fertility. These medicines must be taken with the OCP to prevent menstrual irregularities and adverse effects in pregnancy. The regimen takes ∼6 months to be effective. Women who have more than four cycles of antiandrogens per year are at increased risk of endometrial hyperplasia and endometrial carcinoma. The woman should be monitored regularly for these conditions and plan to have a withdrawal bleed every 2–3 months to reduce these risks.
If endometrial hyperplasia is suspected a transvaginal ultrasound and endometrial biopsy are indicated to confirm the diagnosis especially if prolonged or irregular vaginal bleeding occurs. Hysteroscopy may be needed to determine whether fibroids or polyps are present. Endometrial hyperplasia sometimes resolves spontaneously. If endometrial hyperplasis atypia is present it may progress to endometrial carcinoma, which is induced by oestrogen and has a relatively good prognosis. Treatment depends on the woman. Intrauterine devices are appropriate if contraception is a goal and to control heavy bleeding. High-dose progestins may be indicated to limit endometrial thickening. The ultrasound and/or biopsy should be repeated after 6 months of treatment.
Partners and family need to be involved in explanations about PCOS and its effects as well as management discussions and education programes where relevant so they can support the woman and understand the associated risks. Achievable goals need to be set in consultation with the woman. The effect of having PCOS, its symptoms, and the impact on fertility can generate significant psychological distress, which exacerbates the physical issues, contributes to the diabetes and cardiovascular risk, and reduces quality of life (Coffey & Mason 2003; Wilhelm et al. 2003). Tools such as the K10 or HADs used on a regular basis can help identify the degree and progression of depression and are a helpful guide to treatment (see Chapter 15). Anxiety may be present in addition to depression. Depression needs to be treated, preferably with non-medicine options, because it affects the success of other treatment measures.
Exercise improves mood and fitness. Women with PCOS report significant body image issues, mood disturbances, depression, reduced emotional well being, low quality of life and life satisfaction and a negative impact of PCOS on their sexual self-worth and sexual satisfaction (Janssen et al. 2008). Managing symptoms such as hirsutism, obesity, menstrual irregularity and low fertility can significantly improve their emotional and sexual well being.
Tailored psychotherapy such as cognitive behavioural therapy or self-help programmes such as Mood Gym, and stress management can be beneficial. If medicines are indicated those that reduce PCOS symptoms may be useful. For example, SSRIs to control weight but the woman should be warned that these medicines could affect her sleep.
Pregnancy
Managing diabetes during pregnancy is challenging for the woman concerned and health professionals. Hormones released by the placenta predispose the mother to hyperglycaemia and increase the amount of glucose available to the fetus. Insulin sensitivity increases in the first trimester but insulin resistance develops in the second and third trimesters. The placental glucose transporter, GLUT-1, increases the transplacental glucose flux. The activity of GLUT-4 at the maternal cellular level decreases, which means glucose is not utilised by the mother and high levels cross the placenta where they are utilised by the fetus. This sets the scene for macrosomia and fetal abnormalities and makes vaginal delivery difficult (Hollingsworth 1992).
Prepregnancy counselling is essential to limit risks to mother and baby (Coustan 1997). Women who attend prepregnancy counselling have significantly lower risk of major fetal abnormalities (Ray et al. 2001). It should be provided to all women with diabetes of reproductive age, for example, at each annual review (Australian Diabetes in Pregnancy Society (ADIPS) 2005). Women with Type 2 diabetes and GDM are usually older than those with Type 1, are often overweight, higher parity, and belong to minority ethnic groups (Dornhurst et al. 1992). However, Type 2 diabetes is increasing in prevalence among adolescents and unwanted and unplanned pregnancy rates are high in this age group. In addition women are delaying childbirth, often until the mid-30s or 40s, which carries age-associated risks, and increases the likelihood that diabetes complications will be present and could affect the pregnancy. For example, age appears to be a significant factor in pregnancy-related MI although MI during pregnancy is rare (Karamermer & Ross-Hesselink 2007).
Coordinated multidisciplinary team care that includes as a minimum an obstetrician, endocrinologist, diabetes educator, dietitian, and paeditrician with experience managing diabetes is essential to optimal outcomes. Key management includes strategies to:
- Plan the pregnancy.
- Achieve and maintain HbA1c as close as possible to normal before (<7%), during and after pregnancy.
- Commence folate supplements (5 mg daily).
- Undertake a comprehensive medication review (see Table 14.1). Stop OHA and commence insulin if the woman has Type 2 diabetes. Optimise the insulin regimen in women with Type 1 diabetes. Cease medicines likely to adversely affect the fetus. Commonly used medicines that may be contraindicated in pregnancy include:
- Ace inhibitors.
- B blockers
- Aspirin
- Coumarins
- Calcium channel blockers
- Statins
- NSAIDS
- Caution is required with antidepressants, corticosteroids, and opioids (Gardiner 2002).
- Ask about complementary medicine/therapy (CAM) use. Women frequently use CAM during pregnancy, particularly supplements such as raspberry leaf, ginger and chamomile tea (Foster et al. 2006). They also use massage and essential oils. Women who use CAM are generally well-informed and likely to take proactive self-care, be well educated, non-smokers and primiparous. The proportion of pregnant women with diabetes using CAM is unknown, but CAM use is high in the general diabetic population. While there are many benefits, there are also risks especially in the first trimester, and women with renal and liver disease, see Chapter 8 and Chapter 10. Some women elect to use complementary approaches (CAM) to pain management during labour or supplement conventional pain management strategies with CAM. Tournaire & Theau-Yonneau (2007). Undertook a systematic review of randomised controlled trials, which indicated pain was reduced and women receiving acupuncture and those receiving hydrotherapy required less analgesia. Women were not dissatisfied with conventional pain relief and had different expectations of pain management from health professionals. Strategies such as massage with or without essential oils, which reduce fear also have positive benefits including reducing lower back pain (Alaire 2001). Combination or single herbal medicines are sometimes used to manage pain, for example, motherwort. Women sometimes use herbal medicines during the last 4–5 weeks of pregnancy to prepare the cervix and facilitate delivery, for example raspberry leaf in tablet form and was found to shorten the second stage of labour and fewer women required forceps deliveries (Simpson et al. 2001). CAM use prior to and during labour should be discussed with the obstetrician and/or midwife. Thus, CAM use should be assessed at regular intervals.
- Blood glucose monitoring is essential: target levels are 4.0–5.5 mol/L fasting and <7.0 mmol/L two hours postprandial.
- Undertake a comprehensive diabetes complication and manage existing disease before pregnancy. For example, retinopathy requiring laser therapy, cardiovascular disease. The eyes should be examined through dilated pupils. Women should be aware that pre-existing retinopathy could progress during pregnancy. Pre-existing cardiovascular disease can be exacerbated by pregnancy due to haemodynamic changes and increased cardiac output. Delivery places extra demand on the heart (Karamermer & Ross-Hesselink 2007). Significant coronary artery stenosis should ideally be treated prior to pregnancy. The haemodynamic changes gradually return to normal in 3–6 months (Karamermer & Ross-Hesselink 2007). Kidney function should be checked because women with microalbuminuria at risk of pre-eclampsia and microalbuminuria may progress during pregnancy if significant renal impairment is present and require dialysis (Biesenbach et al. 1992; Rossing et al. 2004), see Chapter 8. Some experts regard significant renal impairment to be a contraindication to pregnancy (ADIPS 2005) particularly given a third of women with severe renal impairment die within ∼16 years (Rossing et al. 2004). Identify autonomic neuropathy-associated conditions such as gastroparesis, hypoglycaemic unawareness and orthostatic hypotension, which make management more difficult, are distressing for the woman and her family and may put her safety and/or life at risk. Autonomic neuropathy may predispose the mother to intractable vomiting and precipitate metabolic disturbances such as ketoacidosis.
- Assess general health and psychological wellbeing. For example detect the presence of comorbidities such as thyroid and coeliac disease in women with Type 1 diabetes, which could complicate the pregnancy.
- Undertake an education review of general diabetes knowledge and provide specific pregnancy and childbirth information. Revise home management of emergencies such as sick days/hyperglycaemia and morning sickness.
- Encourage women who smoke to quit and limit alcohol consumption.
- Perform an ultrasound at 18–20 weeks and if necessary the ultrasound should be repeated to check fetal cardiac status at 24 weeks and fetal growth at 28–30 weeks and again at 34–36 weeks.
- Encourage the woman to eat a healthy well balanced low GI diet to ensure optimal nutrition for herself and the developing fetus. The carbohydrates need to be spread over the day if problems occur. Foods likely to cause listeriosis should be avoided. Dietitian advice is advisable. Listeriosis can be transmitted from the mother to the fetus and cause miscarriage, stillbirth, premature delivery or a very ill baby at birth.
- Encourage participation in regular exercise such as ‘Preggie Bellies’ helps blood glucose control, general fitness, and well being and mental health.
- Encourage participation in childbirth classes.
- Develop and document a plan for managing insulin during delivery and in the immediate post partum period.
- Develop a plan for reviewing the mother’s glycaemic control after discharge and ensuring the woman has adequate support at home.
- Foster mother–child bonding by providing woman-centred holistic care and supporting the woman and her family to adjust to parenthood. Monitoring for the ‘three day blues’ and postnatal depression is essential. For example, Rasmussen et al. (2007) found women with Type 1 diabetes were significantly stressed about the baby’s safety if they had a ‘hypo’ and relied on their mothers and husbands for support. Mutual mother/daughter guilt feelings emerged (the guilt dynamic) where daughters felt guilty about the impact their diabetes had on their mother’s lives and mothers felt guilty because their daughters developed diabetes and they could not prevent it. Chapter 15 describes psychological therapy. In addition, many small studies indicate massage, with and without essential oils, reduces stress, improves sleep for both the mother and baby, facilitates bonding and is safe (Bongaard 2007). Thus, massage may be a useful non-medicine option in high risk groups or could be used in combination with other therapies (Bongaard 2007).
- Specific care of the mother and baby during labour and delivery are specialty areas and outside the scope of this book. Most women should deliver at term and vaginally unless there are medical or obstetric reasons for earlier delivery/Caesarian section.Usually blood glucose is measure every 1–2 hours and usual diet and insulin dose maintained. If a Caesarian section is needed, the procedure should be scheduled for the morning if possible. The dose of long-acting insulin on the evening prior to the surgery may need to be reduced to reduce the risk of hypoglycaemia after delivery. Women with Type 1 diabetes may need an insulin/glucose infusion.
- Women are encouraged to breast feed. Strategies to reduce hypoglycaemia during breastfeeding include having a snack before or while feeding, avoiding caffeine, drinking plenty of water, and having glucose close by to treat hypoglycaemia early if it occurs. Insulin is not contraindicated in breastfeeding.
- Discuss contraception plans prior to discharge.
Comprehensive assessment should be undertaken 1–4 weekly during the first 30 weeks of pregnancy and then weekly until delivery. Routine fetal monitoring should occur and an ultrasound performed to ensure the estimated date of delivery is accurate and develop the delivery plan. In most cases the women will be able to have a normal vaginal delivery unless there are obstetric complications.
Table 14.1 Medication management during pregnancy. A comprehensive medication review should be undertaken regularly in all people with diabetes, when planning a pregnancy and when the woman becomes pregnant. See also Chapter 5 .
| Medication | Management | Potential adverse events |
| Insulin in Type 1 and Type 2 diabetes | Dose should be titrated regularly according to blood glucose monitoring to achieve targets and avoid hypoglycaemia. Insulin aspart has been shown Insulin aspart has been shown to be safe to mother and fetus in Type 1 diabetes (Hod et al. 2007) and achieving postprandial blood glucose control (Pettitt et al. 2007).Glargine has been associated with mitogenic activity and should be used with caution in pregnancy (Hirsch 2005).Women with Type 2 diabetes will need education about managing insulin and hypoglycaemia as well as reassurance. | Hypoglycaemia, especially nocturnal hypoglycaemia during 6–18 weeks gestation.Significant repeated hypoglycaemia should be investigated to ensure there are no underlying disease processes and fetal growth and development monitored.Death, although rare, does occur (Ter Braak et al. 2002).Weight gain. |
| Oral glucose lowering agents (OHA) in Type 2 diabetes | Metformin The overall individual risks need to be considered just those associated with pregnancy. Metformin may be indicated in severe insulin resistance (Simmons et al. 2004). There is limited information about dose adjustment during pregnancy. Sulphonylureas Sulphonylureas cross the placenta, but second generation cross to a lesser extent: glipizide 6.6% and glyburide 3.9% (Feig et al. 2007). Thiazolidinediones (TZD) Rosiglitazone crosses the placenta especially after 10 weeks gestation | A number of studies suggest OHAs do not increase the risk of fetal malformation (Gutzin et al. 2003). Other researchers show metformin is not associated with fetal abnormalities and concentration in breast milk is low (Gilbert et al. 2006). Long-term data are not available thus, caution is recommended. The product prescribing recommends changing to insulin during pregnancy. Feig et al. (2007) found no evidence of fetal abnormalities in animal or human studies if the medicines were used in recommended doses. However, the studies were small and short term and these OHA cannot be recommended for use in pregnancy. |
| However, when a woman with Type 2 diabetes becomes pregnant she should be referred urgently to an endocrinologist and OHAs continued until the transfer to insulin to avoid the risk of hyperglycaemia to the fetus (ADIPS 2005). | ||
| Antihypertensive agents | ACE inhibitors should be ceased during pregnancy. Methyldopa, oxprenolol, clonidine, labetolol, prazosin and nifedipine can be continued safely (Australasian Society for the Study of Hypertension in Pregnancy 2005). The effects of angiotensin 2 blocker is not known | ACE represent a threat to the fetus in the 3rd trimester and their safety is not established in the first trimester. |
| Lipid lowering agents | Statins have been associated with fetal abnormalities and are contraindicated in pregnancy (Edison et al. 2004). | |
| Any other medicine | Check the prescribing information before using. |
Complications of pregnancy
Complications can be reduced by careful monitoring and proactively managing the pregnancy especially controlling blood glucose and other risk factors. The following maternal complications are associated with maternal hyperglycaemia:
- Early: miscarriage, fetal abnormalities, difficulty performing ultrasound.
- Antenatal: hypertension and pre-eclampsia, hypoglycaemia. During delivery: Increased possibility that labour will need to be induced or Caesarian section will be required because of conditions such as macrosomia, shoulder dystocia. Increased risk of perioperative complications if surgery is required. For example hypoglycaemia can have serious consequences in women having epidural anaesthesia because the usual counter-regulatory response is blocked and because pregnant women are more prone to hypoglycaemia. Hypoglycaemia can retard reversal of hypotension in these situations (Marx et al. 1987). However, maternal glucose is often higher during Caesarian section than vaginal delivery (Andersen et al. 1989).
- Postpartum: maternal haemorrhage, infection, thrombosis, hypoglycaemia.
- Deterioration in existing diabetes complication especially renal disease and retinopathy and cardiovascular status. Cardiovascular disease is encountered more frequently because women ore older when they begin their families, a family history of cardiac disease, and have often have diabetes for >10 years. In addition, obesity is more common and smoking increases the risk. An ECG should be performed if chest discomfort develops especially if the woman has known cardiovascular risk factors (Karamermer & Roos-Hesselink 2007). Indications of heart disease include:
- Serious or progressive dyspnoea (mild dyspnoea is usual)
- Syncope on exertion
- Chest pain/discomfort associated with exertion.
- Serious or progressive dyspnoea (mild dyspnoea is usual)
The maternal death rate is comparable to non-diabetic women when care is delivered by a qualified, collaborative multidisciplinary team, ∼0.5% of pregnancies in the UK (Chief Medical Officer’s Report 2000).
Effects of diabetes on the baby
Consequences of maternal hyperglycaemia for the fetus include macrosomia, fetal distress, and birth injuries, which are largely preventable by good obstetric management and controlling maternal blood glucose levels. After delivery the baby may develop hypoglycaemia, hypothermia, hypocalcaemia, transient cardiomyopathy or other problems depending on the intrauterine conditions during pregnancy, delivery, and gestational age. The baby will need intensive monitoring initially, which might affect the bonding process.
Neonatal hypoglycaemia, defined as blood glucose <2.6 mmol/L up to 72 hours after birth, can cause significant morbidity and death if it is not recognised and treated effectively (Western Australian Center for Evidence Based Nursing and Midwifery 2006). The estimated incidence of newborn hypoglycaemia is between 1 and 5 per 1000 live births but may be up to 30% in at-risk infants (Hewitt et al. 2005). Hypoglycaemia is often present at birth and is usually transient in babies of non-diabetic mothers. Infants most at risk are:
- <2 kg or >4 kg at birth
- born before 37 weeks gestation
- <10th percentile for weight (small for gestational age)
- >90th percentile for weight (large for gestational age)
- retarded uterine growth
- born to mother with diabetes or GDM
- those with sepsis effectively (World Health Organisation (WHO 1997; Western Australian Center for Evidence Based Nursing and Midwifery 2006).
The maternal and baby’s blood glucose levels correlate at birth. Thus, the maternal blood glucose level is the most significant determinant of neonatal hypoglycaemia (Andersen et al. 1989). The signs of hypoglycaemia in the neonate are often non-specific and include high pitched crying, hypothermia and poor temperature control, sweating, refusing to feed or poor sucking, exaggerated Moro reflex, irritability, hypotonia, tachyapnoea, tachycardia, cyanosis, and lethargy. All of these signs also occur in other conditions.
Traditionally, hypoglycaemia is diagnosed in the presence of Whipple’s Triad: the presence of characteristic clinical signs of low blood glucose, low blood glucose, resolution of the signs once euglycaemia is re-established. However, the symptoms are unreliable in neonates, as can be seen from the preceding list. Having a high level of suspicion and performing a blood glucose test are essential. Once hypoglycaemia is corrected, babies are usually able to maintain blood glucose levels with normal breastfeeding on demand. Supplemental formula feeds may be required in the first 48 hours. Babies need to be kept warm and closely monitored in the neonatal nursery until they are stable.
Longer term effects of maternal hyperglycaemia on the child
Childhood obesity is increasing in many countries especially in ethnic minorities, see Chapter 1. The factors contributing to childhood obesity and associated consequences (insulin resistance, Type 2 diabetes, and diabetic complications) are debated. Genetic and environmental factors play a role (Dornhurst 2003) including the intrauterine environment during pregnancy, which has lasting effects on the child, the so-called Baker Hypothesis.
The fetus adapts structurally and functionally to reduced availability of essential nutrients. That is, under intrauterine ‘famine’ conditions available glucose is diverted to vital organs such as the brain and away from less immediately vital organs such as the pancreas. Insulin resistance develops in peripheral tissues to ensure a constant supply to vital organs and growth (birth weight) is retarded. The combination of reduced beta cell mass and pre-programming insulin-sensitive tissue to be insulin-resistant confers the risk of future diabetes on susceptible individuals (Baker et al. 1993). Low birth weight may be due to inadequate maternal intake and/or placental dysfunction. The association between Type 2 diabetes and low birth weight has been demonstrated in a number of population-based studies; however, the risk in developed countries with an adequate food supply is low (Boyko 2000) but may be higher in less affluent countries (Gray et al. 2002).
High birth weight is also associated with future risk of impaired glucose tolerance and Type 2 diabetes (Pettitt et al. 1996), which is likely to be a significant factor as the number of overweight women giving birth increases and they deliver higher birth weight babies. High birth weight is primarily due to maternal hyperglycaemia.
Memory deficits in childhood have also been attributed to poorly controlled maternal diabetes during pregnancy (De Boer 2007). De Boer suggested inadequate levels of iron and oxygen during development of the hippocampus (memory centre) in uterocontributed to the deficits, which were significant by age three and a half. She suggested available iron was used to manufacture haemoglobin and diverted away from the hippocampus, which is a metabolically active part of the brain that requires a lot of iron during prenatal neuronal development. The longer term implications of De Boer’s study are unknown and the research is ongoing. Significantly, the deficits were only noticeable on difficult memory tasks. The findings do underscore the importance of prenatal iron supplements and planning pregnancies.
Gestational diabetes
Gestational diabetes (GDM) refers to carbohydrate intolerance of variable severity that first appears during pregnancy (Metzger 1998). GDM is the most common metabolic complication of pregnancy. Insulin resistance and hyperglycaemia develop as a result of the hormones produced by the placenta and declining beta cell function. The hormones oestradiol, cortisol and human placental lactogen rise during pregnancy to ensure that the fetus receives sufficient glucose to grow and develop normally. As a consequence, the mother’s cells become more resistant to insulin and the mother compensates by producing more insulin and using fat stores to produce energy for her own needs. GDM may in fact represent part of the continuum towards Type 2 diabetes.
It is recommended that all pregnant women be screened for diabetes between 24 and 28 weeks gestation, the time the placenta begins to produce large quantities of diabetogenic hormones. The most frequently used screening test is a non-fasting morning glucose challenge using 50 g glucose load. Levels ≥7.8 mmol/L at 1 hour identify 80% of women with GDM (American Diabetes Association 2004) or ≥8 mmol/L at one hour if 75 g glucose load is used. If the screening test is positive, a diagnostic fasting OGTT is usually performed using 75 g glucose solution. Diagnostic levels are baseline ≥5.5 mmol/L and ≥8 mmol/L at 2 hours (Hoffman et al. 1998) (100 g is used in the US). Women who do not meet the diagnostic criteria for GDM may still have babies with glucose-related macrosomia, respiratory distress, hyperbiluribinaemia, hypoglycaemia and require admission to neonatal intensive care. Adverse pregnancy outcome such as stillbirth, Caesarian section, and pre-eclampsia can also occur.
Usually the blood glucose returns to normal after the baby is delivered. However, approximately 40% of women with gestational diabetes will develop diabetes in later life. In addition, the baby is at increased risk of Type 2 diabetes. In most cases the OGTT is repeated 6–8 weeks after delivery and then on regular basis, for example, yearly, although many women do not return for follow up. A recent study of women with GDM in Sweden between 1995 and 2005 (n = 385) were tested for beta cell autoantibody markers to determine their risk of developing Type 1 diabetes (Nilsson et al. 2007). Six per cent tested positive for at least one islet cell antibody: ICA, GAD or IA-2A. These women were followed and autoantibody levels reanalysed. On OGGT was performed in women who did not develop diabetes. Fifty per cent of the autoantibody positive women developed Type 1 diabetes and 21% had IFG or IGT but none had developed Type 2 diabetes. The overall numbers in the study were small, but autoantibody testing may be indicated in some women who develop GDM.
Who is at risk from gestational diabetes?
Gestational diabetes can occur in any pregnancy, however, those women at highest risk are categorised as:
- Older then 30 years.
- Having a family history of diabetes or previous gestational diabetes or having had a large baby previously.
- Belonging to an at-risk ethnic group, for example, Vietnamese, Asian Indians, Australian Aboriginals.
- Being obese >12% of ideal bodyweight and having other features of the metabolic syndrome, see Section 1.6.
Diet and exercise are first line treatment of GDM to control maternal blood glucose and supply essential nutrients for normal fetal growth and development. Generally low GI foods are recommended where 40–50% calories come from complex high fibre carbohydrate, 20% from protein and 30–40% from unsaturated fats distributed evenly throughout the day. Nutrition counselling by a dietitian is recommended. Blood glucose goals are fasting <5.5 mmol/L and 1 hour postprandial <8 mmol/L. Insulin is indicated if these targets cannot be met.
Calorie restriction can predispose the woman and the fetus to ketonaemia and reduce psychomotor development and IQ between the 3rd and 9th year of age in the child especially during the second and third trimesters (Rizzo et al. 1995). Pregnant women who remain active have a 56% lower risk of developing glucose intolerance and GDM than inactive women (Zhang et al. 2006) and lower risk of Type 2 diabetes in the longer term (Artal & O’Toole 2003; Ceysens et al. 2007). There are no data for optimal weight gain during pregnancy (Metzger et al. 2007). The Fifth International Workshop-Conference on GDM (2003) recommended a relatively small weigh increase during pregnancy: ∼7 kg for obese women and up to 18 kg in underweight women.
Women at risk of GDM are advised to monitor their fasting and 2-hour postprandial blood glucose levels after each meal although there is no objective evidence to support the recommended frequency. CGM may be used to obtain a blood glucose pattern in some women, see Chapter 3. The aim of treatment is to keep blood glucose within the normal range (3–6.5 mmol/L), ensure the diet is appropriate, provide good obstetric care, and deliver the baby as close to term as possible.
Insulin will be required during pregnancy if blood glucose cannot be controlled using diet and exercise, generally when the fasting glucose is >7 mmol/L on three consecutive occasions but the duration of time on diet/exercise therapy before commencing insulin is unknown. Buchanan et al. (1994) recommended using insulin if the fetal abdominal circumference is >75th percentile for gestational age on ultrasound. However, a limitation of this method is that it provides a ‘snapshop’ rather monitoring continuous longitudinal fetal growth and should not be used alone especially given fetal macrosomia is related to maternal blood glucose levels. Insulin analogues are generally used, see Chapter 5 and the starting dose is calculated according to the woman’s weight generally ∼0.8 units/kg (non-obese), 0.9–1.0 units/kg (overweight and obese respectively).
Oral hypoglycaemic agents (OHAs) are contraindicated because they cross the placenta and cause neonatal hypoglycaemia and may have other unknown effects. However, metformin is used in Europe and South Africa and glibenclamide in South Africa without any reported fetal or maternal adverse outcomes. Although most other countries do not recommend OHA use during pregnancy (Metzger et al. 2007) the Metformin in Gestational Diabetes (MiG) (Rowan 2007) set up to compare metformin with insulin on perinatal outcomes suggests metformin is safe in the short term but may be contraindicated if the baby is small, or if the mother has pulmonary oedema or sepsis due to the risk of lactic acidosis, see Chapter 7. Metformin reduces absorption of vitamin B12 but this can be overcome by supplementing with calcium.
Insulin is given as required during labour. Some obstetric units use insulin infusions to control hyperglycaemia, which may be exacerbated by the pain and stress of labour. Frequent blood glucose monitoring is required because insulin requirements fall dramatically after delivery and maternal hypoglycaemia is possible. The baby is prone to hypoglycaemia after delivery and should be monitored closely for 24 hours as indicated in the preceding section.
Encourage breastfeeding. Breastfeeding provides essential nutrition and immunity for the baby. It decreases the risk of obesity and Type 2 diabetes in later life in babies of women who develop GDM.
Type 1 diabetes
As stated, planning the pregnancy and achieving optimal health before becoming pregnant significantly improves outcomes. Insulin requirements vary throughout the pregnancy and frequent blood glucose monitoring is essential so doses can be adjusted appropriately.
The specific insulin dose depends on individual characteristics. Overweight women may be less sensitive to insulin and may require larger doses than lean women. NovoRapid is approved for use in pregnancy. There do not appear to be any safety issues using the other insulin analogues in pregnancy but clinical experience with Apidra and Detemir is not as extensive with the other analogues (Nankervis 2008 oral presentation).
Blood glucose testing before and one hour after each meal is ideal to help titrate insulin doses as needed. Insulin requirements usually increases by ∼20% in the first 5–6 weeks due to increased levels of progesterone and continues to rise slowly to weeks 9–11 at which time the placenta begins to produce progesterone and ovarian production ceases (Jovanovic 2004).
Progesterone levels may decline temporarily during the switch and consequently insulin requirements can drop especially in lean insulin-sensitive women. Hypoglycaemia risk increases. It may be necessary to perform 3 am blood glucose tests to determine whether hypoglycaemia occurs during the night. Progesterone levels increase again after ∼8–10 days and insulin requirements begin to increase and continue to do so during each trimester. At term when contractions begin insulin requirements drop because the available glucose is utilised to support uterine contractions. Three am blood glucose testing may be required again at this time.
After delivery insulin requirements generally drop significantly and only very low doses may be needed in the first 24–48 hours and eventually return to prepregnancy requirements. However, breastfeeding women usually require less long acting insulin because blood glucose is used to produce milk, but they may need more short-acting insulin at mealtimes. Hyperglycaemia may mean extra lactose in breast milk and cause problems for the baby such as diarrhoea and putting on excess weight.
Women with Type 1 diabetes may have hypertension as a consequence of nephropathy or hypertension may develop as a consequence of the pregnancy (American Diabetes Association 2000). Blood pressure must be carefully monitored and controlled to reduce the risk of pre-eclampsia, progression of renal disease and retinopathy. Medicines management can be complication because ACE inhibitors, β blocking agents and diuretics are generally contraindicated.
Type 2 diabetes
Women with Type 2 diabetes and hyperglycaemia may be commenced on insulin prior to conceiving to optimise blood glucose levels and improve pregnancy outcomes.
Women with Type 2 diabetes are usually commenced on insulin during pregnancy. As with Type 1 diabetes insulin requirements slowly increase over the course of the pregnancy. However, the typical temporary drop seen ∼9–11 weeks in women with Type 1 diabetes may not occur because women with Type 2 diabetes are generally insulin resistant. Insulin requirements are affected by hormone levels but also by arbohydrate intake and activity levels.
Women with Type 2 diabetes should monitor their blood glucose with the same frequency as those with Type 1 but are generally less prone to nocturnal hypoglycaemia.
After delivery women who were treated with diet and exercise may be able to resume that regimen but insulin is recommended if the woman decides to breast feed (ADIPS 2005). OHAs can be used but metformin is present in breast milk and the baby may develop hypoglycaemaia. Therefore, regular blood glucose testing may be needed, but could be stressful for the parents.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree