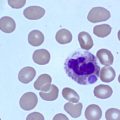
Leukocytosis is one of the most common laboratory abnormalities in medicine, and one of the most frequent reasons for hematologic consultation. Effective evaluation of leukocytosis requires an attentive history, careful physical examination, meticulous review of the complete blood count and peripheral blood smear, judicious application of laboratory and radiologic testing, and thoughtful analysis. Definitive diagnosis may require bone marrow aspiration and biopsy, imaging studies, and specialized molecular tests. The differential diagnosis of leukocytosis includes physiologic responses to a broad range of infectious and inflammatory processes, as well as numerous primary hematologic disorders such as leukemias, lymphomas, and myeloproliferative neoplasms.
Leukocytosis is an increase of the white blood cell (WBC, or leukocyte) count; for adults, this is usually more than 11,000/μL. The term leukocyte refers collectively to granulocytes (neutrophils, eosinophils, and basophils), monocytes, and lymphocytes. Any of these individual lineages, or combinations of them, may account for increased WBC.
Leukocytosis is one of the most commonly encountered laboratory abnormalities in clinical medicine, and is a frequent reason for both outpatient and inpatient consultation by a hematologist. Leukocytosis may be an acute or chronic process. It is most often caused by an appropriate physiologic response of normal bone marrow to an infectious or inflammatory stimulus. Less frequently, leukocytosis is caused by a primary bone marrow disorder, such as leukemia, lymphoma, or a myeloproliferative neoplasm. Defining the cause of leukocytosis demands a thorough clinical history, physical examination, and review of the peripheral blood smear, and may require additional laboratory testing, radiologic imaging, bone marrow examination, and molecular or cytogenetic analyses.
White blood cell (WBC) development
Three-quarters of all nucleated cells in the bone marrow are committed to the production of leukocytes. Each day, approximately 1.6 billion leukocytes are produced per kilogram of body weight, and more than half are neutrophils.
The peripheral WBC count is an indirect measure of the body’s total mass of leukocytes, because only 2% to 3% of total leukocytes circulate in the bloodstream; 90% of WBCs are present in bone marrow stores and 7% to 8% are stored in other tissues. The WBC count, as measured in a peripheral blood complete blood count (CBC), is influenced by changes in: (1) the size of the bone marrow storage pool; (2) the rate of WBC release from storage pools; (3) the balance of actively circulating WBC versus reversibly adherent (marginated) cells; and (4) rates of migration to, and consumption of, leukocytes in peripheral tissues.
Development, maturation, and survival of granulocytes
Despite their distinctive appearances, functions, and patterns of gene/protein expression, all cellular elements of peripheral blood, that is, red blood cells (RBCs), platelets, and WBCs, ultimately arise from hematopoietic stem cells (HSCs). This rare population of bone marrow cells (<0.2%) has the ability to both self-renew and give rise to more differentiated committed progenitor cells, which are committed to specific cellular lineages, including precursors to granulocytes, monocytes, lymphocytes, RBCs, and megakaryocytes. HSCs and progenitor cells are mononuclear cells morphologically indistinguishable from lymphocytes, but they can be identified in the bone marrow by their expression of antigens such as CD34.
Differentiating granulocytes pass through 6 successive, morphologically distinct stages of maturation. The earliest recognizable form is the myeloblast, followed in turn by promyelocytes and myelocytes; together, these cells constitute the proliferative pool of myeloid cells. The later stages of neutrophil maturation, that is, metamyelocytes, bands and, ultimately, polymorphonuclear neutrophils (polys), are postmitotic. Maturation of granulocytes is marked by nuclear condensation and eventual segmentation (hence the term polymorphonuclear), and acquisition of cytoplasmic granules, which contain pools of degradative enzymes and other products required for effective bacterial killing.
The bone marrow reserve of maturing neutrophils represents approximately a 1-week supply. This storage pool allows a rapid response to demand for WBCs and can triple the level of circulating leukocytes within hours. Neutrophils that leave the marrow can circulate, marginate, or enter peripheral tissues. Margination is the process whereby neutrophils reversibly adhere to the blood vessel wall using specialized adhesion molecules. When stimulated by infection, inflammation, drugs, or metabolic toxins, these cells can demarginate to enter the pool of freely circulating leukocytes. Granulocytes remain in circulation or in peripheral tissues for only a few hours before cell death. The estimated total life span of maturing granulocytes is 11 to 16 days, most of which involves bone marrow maturation and storage.
Based on the staining qualities of their cytoplasmic granules, 3 major granulocytic subgroups are recognized. Most granulocytes are neutrophils (50%–70% of circulating leukocytes), whereas eosinophils and basophils typically constitute only 1% to 2% each. The specialized contents of their distinctive granules support their important roles in bacterial killing (neutrophils), parasitic infections (eosinophils), and viral infections or allergic immune reactions (basophils).
Development, maturation, and survival of granulocytes
Despite their distinctive appearances, functions, and patterns of gene/protein expression, all cellular elements of peripheral blood, that is, red blood cells (RBCs), platelets, and WBCs, ultimately arise from hematopoietic stem cells (HSCs). This rare population of bone marrow cells (<0.2%) has the ability to both self-renew and give rise to more differentiated committed progenitor cells, which are committed to specific cellular lineages, including precursors to granulocytes, monocytes, lymphocytes, RBCs, and megakaryocytes. HSCs and progenitor cells are mononuclear cells morphologically indistinguishable from lymphocytes, but they can be identified in the bone marrow by their expression of antigens such as CD34.
Differentiating granulocytes pass through 6 successive, morphologically distinct stages of maturation. The earliest recognizable form is the myeloblast, followed in turn by promyelocytes and myelocytes; together, these cells constitute the proliferative pool of myeloid cells. The later stages of neutrophil maturation, that is, metamyelocytes, bands and, ultimately, polymorphonuclear neutrophils (polys), are postmitotic. Maturation of granulocytes is marked by nuclear condensation and eventual segmentation (hence the term polymorphonuclear), and acquisition of cytoplasmic granules, which contain pools of degradative enzymes and other products required for effective bacterial killing.
The bone marrow reserve of maturing neutrophils represents approximately a 1-week supply. This storage pool allows a rapid response to demand for WBCs and can triple the level of circulating leukocytes within hours. Neutrophils that leave the marrow can circulate, marginate, or enter peripheral tissues. Margination is the process whereby neutrophils reversibly adhere to the blood vessel wall using specialized adhesion molecules. When stimulated by infection, inflammation, drugs, or metabolic toxins, these cells can demarginate to enter the pool of freely circulating leukocytes. Granulocytes remain in circulation or in peripheral tissues for only a few hours before cell death. The estimated total life span of maturing granulocytes is 11 to 16 days, most of which involves bone marrow maturation and storage.
Based on the staining qualities of their cytoplasmic granules, 3 major granulocytic subgroups are recognized. Most granulocytes are neutrophils (50%–70% of circulating leukocytes), whereas eosinophils and basophils typically constitute only 1% to 2% each. The specialized contents of their distinctive granules support their important roles in bacterial killing (neutrophils), parasitic infections (eosinophils), and viral infections or allergic immune reactions (basophils).
Development of monocytes
Monocytes develop from a granulocyte-monocyte precursor that also gives rise to the granulocyte lineage. Under the influence of specialized cytokines, monocytes transform into macrophages that reside in peripheral tissues. Monocytes are the largest mature cells in circulation, and play major roles in phagocytosis and regulation of the immune response.
Lymphocyte development
There are 2 major categories of lymphocytes, B cells and T cells, and lesser lymphocyte populations, including natural killer (NK) cells. B lymphocytes are responsible for generating the enormous range of highly specific antibodies (immunoglobulins) that are required for effective immune function. B cells arise in the bone marrow, rearrange their immunoglobulin genes in lymph nodes after antigen exposure, and take up residence in lymph nodes. A subset of B lymphocytes further matures into plasma cells, which primarily reside in bone marrow and generate large quantities of immunoglobulins in response to infectious or inflammatory challenges.
T lymphocytes originate in the bone marrow, mature in the thymus, and take up residence in lymph nodes, bone marrow, and peripheral tissues. Cytotoxic T cells can directly attack abnormal or infected cells (ie, bearing viral antigens on their cell surface), and modulate the activity of B cells and other aspects of the immune system. T-helper cells secrete cytokines that augment specific immune responses. By contrast, T-suppressor or regulatory cells secrete cytokines that can dampen the intensity of immune response after the infectious agent is cleared. NK cells, a subpopulation of lymphocytes, lack most of the recognizable surface markers of mature T or B cells. NK cells can nonspecifically target cancer cells or microorganisms. The term large granular lymphocytes (LGLs) usually refers to NK cells, because up to 75% of LGLs function as NK cells.
Evaluation of leukocytosis
The clinical evaluation of leukocytosis is influenced by (1) the nature of the cells involved, (2) the duration of the leukocytosis, and (3) the presence of associated clinical findings. The differential count from a CBC and review of the peripheral blood smear will define whether the increased leukocytes are predominantly granulocytes or lymphocytes, detect abnormalities of WBC morphology, and identify abnormalities of other lineages (eg, anemia, polycythemia, or platelet abnormalities).
The duration of leukocytosis, that is, hours to days versus weeks, months, or years, influences the likely underlying cause. A short duration of leukocytosis suggests an acute event, such an infection or acute leukemia. By contrast, long-standing leukocytosis may represent chronic inflammatory states or hematologic malignancies, such as chronic leukemias or lymphomas. Similarly, the presence of associated clinical findings may point to an underlying systemic illness or reflect consequences of a primary hematologic disorder.
Classification of leukocytosis
The following terms refer to the level and nature of leukocytosis, but do not have strict definitions and may be applied loosely by clinicians. Left shift refers to an increased percentage of immature granulocyte forms in the peripheral blood, which may exhibit toxic granulations (prominent primary granules) and Döhle bodies (prominent secondary granules) in response to severe infections. The presence of myelocytes or even less mature granulocyte forms in peripheral blood should raise the question of an underlying hematologic malignancy or severe trauma.
Leukemoid reactions represent exaggerated leukocytosis (typically 50,000–100,000/μL) and may include in the peripheral blood all recognizable stages of neutrophil maturation, that is, from myeloblasts to mature granulocytes. Leukemoid reactions typically last hours to days and may be caused by either benign or malignant conditions. A leukoerythroblastic reaction caused by myelophthisis is similar (but the total WBC does not need to be high) and also includes nucleated RBCs. Leukoerythroblastosis indicates severe disruption of the marrow by overwhelming infection, myelofibrosis, or bone marrow invasion by cancer, and may be associated with extramedullary hematopoiesis. A leukoerythroblastic reaction in infants can occur with severe hemolytic anemia (eg, erythroblastosis fetalis) or the rare bone disorder, osteopetrosis, in which failure of osteoclasts to resorb bone causes loss of hematopoietic marrow space and resultant extramedullary hematopoiesis.
Hyperleukocytosis refers to a WBC count greater than 100,000/μL, and is seen almost exclusively in leukemias and myeloproliferative disorders. Leukostasis, or sludging of WBC in small vessels of the brain, lungs, and kidneys, is an oncologic emergency that may cause life-threatening cerebral infarcts, cerebral hemorrhage, or pulmonary insufficiency caused by impaired blood flow. Leukostasis is more common in acute myelogenous leukemia than in acute lymphoblastic leukemia, because myeloblasts are larger and more adhesive than lymphoblasts; it is rarely seen in chronic leukemias, even with extremely high WBC counts.
Distinguishing a primary hematologic disorder from a reactive (secondary) leukocytosis
When evaluating leukocytosis, attempts should be made to distinguish a primary hematologic disorder (such as leukemias and myeloproliferative neoplasms) from a secondary effect, that is, the response of normal bone marrow to an infectious or inflammatory challenge. A careful history and physical examination, review of the peripheral blood smear, and laboratory studies are important for making this distinction. Ultimately bone marrow examination, including morphology, chromosome analysis, molecular testing, and imaging studies may be required to conclusively distinguish between these categories of leukocytosis.
True Leukocytosis Versus Pseudoleukocytosis
Leukocytosis may result from (1) increased production, (2) mobilization of storage pools, (3) reduced adhesion to vascular endothelium, (4) decreased migration to peripheral tissues, (5) increased cell survival, or (6) combinations of these processes. Pseudoneutrophilia may result from granulocyte demargination due to exercise, epinephrine, or anesthesia. Because the normal spleen retains a large number of leukocytes, asplenia is associated with an increased WBC count. Corticosteroids, which demarginate granulocytes, decrease neutrophil release from the marrow, and reduce neutrophil egress from the circulation, frequently cause leukocytosis.
Causes of secondary leukocytosis
Infections
Bacterial infections typically cause mild to moderate leukocytosis (11,000–30,000/μL), with a preponderance of mature neutrophils and bands ( Box 1 ). The granulocytosis is usually of short duration, and may be associated with a left shift and toxic granulations or Döhle bodies. WBC counts may transiently decline early in the course of overwhelming sepsis, only to increase later. Some patients with Clostridium difficile infection or tuberculosis may manifest a leukemoid reaction with a WBC count greater than 50,000/μL. Conversely, typhoid fever, brucellosis, tularemia, rickettsial diseases, ehrlichiosis, leishmaniasis, and some cases of Staphylococcus aureus infection may be associated with leukopenia. Viral infections do not typically cause neutrophilia, but leukocytosis may be observed in the early phases of viral infection.
- 1.
Secondary to other disease entities
- a.
Infection
- i.
Acute via release from marginated and storage pools
- ii.
Chronic via increased myelopoiesis (eg, tuberculosis, fungal infection, chronic abscess, other chronic infections)
- i.
- b.
Chronic inflammation
- i.
Rheumatic disease: juvenile rheumatoid arthritis, rheumatoid arthritis, Still disease, and others
- ii.
Inflammatory bowel disease
- iii.
Granulomatous disease
- iv.
Chronic hepatitis
- i.
- c.
Cigarette smoking
- d.
Stress
- e.
Drug induced
- i.
Corticosteroids
- ii.
β-Agonists
- iii.
Lithium
- iv.
Recombinant cytokine administration
- v.
Administration of inhibitors of adhesion molecules
- i.
- f.
Nonhematologic malignancy
- i.
Cytokine-secreting tumors (lung, tongue, kidney, urothelial tumors)
- ii.
Marrow metastasis (myelophthisis)
- i.
- g.
Marrow stimulation
- i.
Hemolytic anemia, immune thrombocytopenia
- ii.
Recovery from marrow suppression
- iii.
Recombinant cytokine administration
- i.
- h.
Postsplenectomy
- a.
- 2.
Primary hematologic etiology
- a.
Congenital neutrophilia
- i.
Hereditary neutrophilia
- ii.
Chronic idiopathic neutrophilia
- iii.
Down syndrome
- iv.
Leukocyte adhesion deficiency (LAD): LAD I and LAD II
- i.
- b.
Acquired hematologic neoplasms
- i.
Acute myelogenous leukemia
- ii.
Myeloproliferative neoplasms
- 1.
Chronic myelogenous leukemia
- 2.
Polycythemia vera
- 3.
Essential thrombocytosis
- 4.
Idiopathic fibrosis
- 1.
- i.
- a.
Infectious lymphocytosis (generally 20,000–50,000/μL small, mature-appearing lymphocytes) is mainly a disease of children. It may be related to coxsackievirus A or B6, echovirus, and adenovirus 12, and is rarely associated with splenomegaly or lymphadenopathy. Infection with Epstein-Barr virus (EBV) can cause atypical lymphocytosis (large and reactive lymphocytes with abundant basophilic cytoplasm) and lymphadenopathy. Human T-lymphotropic virus type 1 (HTLV-1) may produce a transient lymphocytosis (usually <20,000/μL) with fever, rash, and lymphadenopathy. In contrast to most other bacterial infections, pertussis (whooping cough) is frequently accompanied by lymphocytosis.
Noninfectious Causes of Leukocytosis
Leukocytosis can be caused by a variety of malignancies, chronic inflammatory conditions, medications, splenectomy, and in association with hemolytic anemia. Postsplenectomy leukocytosis may last weeks to months and has no clinical importance, but may lead to evaluation for other sources of abnormality. Patients with hemolytic anemia may experience a nonspecific increase in leukocyte production and release, in parallel with the increase in RBC production. Chronic smokers may exhibit a mildly increased WBC count that can persist for years.
Chronic inflammatory conditions, including rheumatoid arthritis, juvenile rheumatoid arthritis, and Still disease; inflammatory bowel disorders, such as Crohn disease and ulcerative colitis; vasculitides; granulomatous infections; and chronic hepatitis are often associated with leukocytosis. These processes are associated with increased expression of cytokines that stimulates neutrophil and monocyte production, but can deplete mature neutrophil pools over time. Leukocytosis associated with these inflammatory conditions is typically more modest than in acute infection or inflammation.
Neutrophilia may occur as a result of physical and emotional stress. This transient increase in circulating neutrophils occurs within minutes of exercise, surgery, or other forms of stress, and typically reverses within hours of elimination of the trigger. Stress leukocytosis is presumed to be caused by catecholamine-induced demargination of neutrophils, and some cases can be prevented by pretreatment with β-adrenergic antagonists. Exercise-induced neutrophilia, however, is not blocked by propranolol, and may be due to redistribution of neutrophils from the lungs. An increased WBC count may be seen in the setting of acute myocardial infarction, but whether this is a risk factor for cardiac ischemia or a result of inflammation is unclear.
Drug Effect
Medications commonly associated with leukocytosis include corticosteroids, lithium, and β-agonists. Steroid administration typically leads to decreased egress from the circulation and increased demargination. Lithium causes neutrophilia by increasing the production of endogenous colony-stimulating factors (CSFs). The cytokines, granulocyte CSF (G-CSF) and granulocyte-macrophage CSF (GM-CSF), are routinely used to mobilize hematopoietic stem and progenitor cells for autologous and allogeneic hematopoietic stem cell transplantation, and can cause pronounced neutrophilia if not appropriately managed. Mobilization is achieved within hours of administration of certain chemokines (interleukin-8), small-molecule antagonists of the CXCR4 receptor (eg, AMD3100), or a small-molecule antagonist of VLA-4 (BIO4860).
Almost any kind of malignancy may cause leukocytosis, as tumors nonspecifically stimulate the production of leukocytes in bone marrow. Some tumors (eg, lung, tongue, kidney, bladder) can secrete G-CSF as an ectopic hematopoietic growth factor. Other tumors (eg, lung, stomach, breast) can cause a leukoerythroblastic reaction when they spread to bone marrow. Patients with Hodgkin lymphoma typically have mild to moderate neutrophilia, but neutrophilia can be associated with many nonhematologic malignancies.
Recovery of cell counts after marrow suppression, as in the case of chemotherapy, may cause rebound leukocytosis that can last for days to weeks. Similarly, hemolytic anemia and idiopathic thrombocytopenic purpura can result in generalized stimulation of the bone marrow and result in a so-called spillover leukocytosis.
Lymphocytosis
Lymphocytes normally represent 20% to 45% of circulating WBCs. Lymphocytosis conventionally refers to a lymphocyte count greater than 4000/μL, and is the second most common cause of leukocytosis ( Box 2 ). The lymphocyte count increases in certain acute and chronic infections. Marked lymphocytosis is observed in individuals infected with pertussis (≥40,000/μL), and lymphocyte counts greater than 100,000/μL indicate a poor prognosis. Chronic brucellosis and syphilis infections may occasionally cause an atypical lymphocytosis.