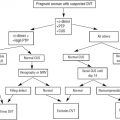
Evaluation and treatment of hematologic disorders in pregnancy requires an understanding of normal physiologic changes during pregnancy. Hematologic disorders may be caused by preexisting conditions, normal physiologic changes, or can be acquired. A multidisciplinary approach is often necessary for monitoring and treatment of both the mother and the fetus. In general, outcomes are good for both the mother and the fetus.
An understanding of hematologic disorders in pregnancy requires knowledge of the normal physiologic changes in pregnancy ( Table 1 ). During pregnancy, plasma volume increases by about 50%, rapidly by week 6 of gestation and then more gradually, peaking by week 30. Erythrocyte mass increases by only 18% to 30% during pregnancy, resulting in a dilution effect (hematocrit 30%–32%) referred to as physiologic anemia of pregnancy. Other physiologic changes, including an increase in clotting factors, occur as the parturient accommodates a growing uterus and placenta and prepares for the hemostasis needed for delivery.
| Test | Reference Intervals | Change in Pregnancy |
|---|---|---|
| CBC | ||
| WBC | 3.00–10.5 × 10 9 /L | ↑ to 10–16 × 10 9 /L |
| Hb | 115–155 g/L | ↓ 100–130 g/L |
| Platelet count | 100–400 × 10 9 /L | ↓ near term to as low as 100 × 10 9 /L |
| PTT | 24–36 s | ↔ |
| INR | 0.9–1.2 | ↔ |
| Fibrinogen | >2.0 g/L | ↑↑ |
| VWF | Group 0: 0.40–1.75 U/mL Nongroup 0: 0.70–2.10 U/mL | ↑ |
| Factor VIII | 0.6–1.95 U/mL | ↑ |
| D-dimer | <300 μg/L | ↑ |
| Protein C | Functional: 0.75–1.60 U/mL Antigen: 0.70–1.20 U/mL | ↔ |
| Protein S | Functional: 0.50–1.00 U/mL Antigen: 0.57–1.20 U/mL | ↓ |
| AT | 0.80–1.25 U/mL | ↔ |
| Homocysteine | <10 μmol/L | ↓ |
| Ferritin | — | ↑ |
| ESR | — | ↑ |
Anemia
In pregnancy, anemia is generally well tolerated. The Centers for Disease Control and Prevention defines anemia in pregnancy as a hemoglobin of less than 11 g/dL in the first and third trimesters and less than 10.5 g/dL in the second trimester. For women with adequate iron stores, the hemoglobin should return to normal around 1 to 6 weeks after delivery. A hemoglobin of less than 10 g/dL should prompt a work-up for a pathologic cause. Severe anemia, defined as a hemoglobin less than 6 g/dL, has been associated with reduced amniotic fluid volume, fetal cerebral vasodilatation, and nonreassuring fetal heart rate tracings. There have also been reports of increased risk of prematurity, spontaneous abortion, low birth weight, and fetal death. A hemoglobin of less than 7 g/dL increases the risk of maternal mortality. Current recommendations are for all pregnant women to have a baseline complete blood count before pregnancy or at the first prenatal visit, and again in the third trimester.
Iron Deficiency Anemia
Iron deficiency anemia accounts for most (>90%) nonphysiologic anemias. During a normal singleton pregnancy, a woman loses 1000 mg of iron to the fetus and placenta, expansion of red blood cells (RBCs), and insensible losses. Studies of the efficacy of iron supplementation on pregnancy outcomes are lacking. Transferrin is usually increased with pregnancy. Serum ferritin is a useful screening test for iron deficiency in pregnancy, with a sensitivity of 90% and specificity of 85%. Iron repletion recommendations are generally the same for pregnant patients as nonpregnant patients. The current recommendation is 15 to 30 mg daily of supplemental elemental iron for all pregnant women. Parental iron can be used for patients who do not absorb or are intolerant of oral iron. Iron sucrose in pregnancy has better safety data and is preferable to iron dextran. Recombinant erythropoietin has been shown to be of some benefit in patients with an inadequate response to iron, but it may cause a hypertensive effect. However, data are limited to only 1 study and this should not be considered standard of care at this point. In patients refractory to standard iron repletion, another cause of anemia should be investigated.
Macrocytic Anemias
Macrocytic anemias are most commonly caused by folate deficiency and, rarely, vitamin B 12 deficiency. Accurate diagnosis of both folate and B 12 deficiency in pregnancy usually requires measuring plasma homocysteine and methylmalonic acid levels. Folate deficiency is caused by increased demands from the fetus and erythropoiesis from the expansion of maternal red cell mass. Hormonal changes of pregnancy can decrease folate absorption and increase urine losses. Folate requirements increase from 50 μg/d in the nonpregnant patient to 150 μg/d in pregnant patients. The current recommendation is 1 mg daily of supplemental folate for all pregnant women. This folate supplementation prevents neural tube defects and folic acid deficiency. Higher doses of folate are recommended in patients on antiepileptic drugs, which further deplete maternal folate levels, and in patients with a previously affected child with a neural tube defect. Repletion for both vitamin B 12 and folate deficiency are the same as for nonpregnant patients. Other less common causes of macrocytic anemia include alcohol use, medications, hypothyroidism, and liver disease.
Hemoglobinopathies
For most women with hemoglobinopathies, pregnancy is possible and successful with increased monitoring to avoid maternal and fetal complications.
Sickle Cell Disease
Sickle cell syndrome is caused by an inherited single nucleotide (GAG/GTG) mutation in the β-globin gene. Heterozygosity for HbS (sickle cell trait) does not cause disease but homozygous inheritance or compound heterozygous inheritance with another mutant β-globin gene does result in sickle cell disease. At least 17 hemoglobinopathies resulting in sickle cell disease variants exist. The more common types are listed in Table 2 . Some risks to the mother and fetus are caused by pregnancy. Pregnancy-related increases in adhesion and coagulation proteins, such as Von Willebrand factor, fibrinogen, and factor VIII, may exacerbate RBC adhesion leading to RBC and platelet aggregation. This process results in occlusion of the vessels and a vasoocclusive event, which may be more frequent during pregnancy. The frequency of obstetric complication in women with sickle cell disease is shown in Table 3 . Sickle cell disease carries approximately a 6.5% risk of spontaneous abortion according to the Cooperative Study of Sickle Disease. Rates of intrauterine growth restriction may be increased in women with sickle cell disease, especially those with acute complications of sickle cell disease during pregnancy. Preterm labor, placental abruption, and preeclampsia may be more common in patients with sickle cell anemia (HbSS disease) compared with healthy African American women without sickle cell disease.
| Disease | Baseline Hb (g/dL) | MCV | Baseline Reticulocyte (%) | Relative Clinical Severity |
|---|---|---|---|---|
| HbSS | 6.0–9.0 | Normal | 5–30 | ++++ |
| HbS-β°-thalassemia | 6.0–9.0 | Low | 5–30 | ++++ |
| HbSC | 10.0–13.0 | Normal | 3–4 | +++ |
| HbS-β + -thalassemia | 10.0–14.0 | Low | 3–4 | ++ |
| Hb AS | 14.0–16.0 | Normal | 0–1 | 0 |
| Transfusion Study, 1978–1986 | Cooperative Study, 1979–1986 | ||||
|---|---|---|---|---|---|
| Control | SS | SC | Sβthal | ||
| Number of pregnancies | 8981 | 100 | 66 | 23 | 225 |
| Gestational age at delivery (wk) | 40 | 37.5 | 38.6 | 37.1 | 37.7 |
| Preterm labor (%) | 17 | 26 | 15 | 22 | 9 |
| Placenta previa (%) | 0.4 | 1 | 2 | 4 | — |
| Abruptio placenta (%) | 0.5 | 3 | 2 | 4 | — |
| Toxemia (%) | 4 | 18 | 9 | 13 | 11 |
| Cesarean section (%) | 14 | 29 | 30 | 26 | — |
Patients should be followed in a high-risk clinic in conjunction with a health care provider familiar with the care of patients with sickle cell disease. A complete blood count, reticulocyte count, urinalysis, and blood pressure monitoring are recommended with each visit. A prenatal vitamin without iron and with additional folate should be prescribed. During the first trimester, preventing nausea and volume depletion may decrease sickle cell–related complications. Close monitoring for the development of sickle cell pain events such as acute chest syndrome, acute splenic sequestration, splenic infarct, and acute multiorgan failure is recommended during pregnancy, labor, and the postpartum period. Pain crises are treated similarly to nonpregnant patients and are outlined in Table 4 . Adequate intravenous fluid administration and oxygenation should be maintained during labor. Analgesia doses may exceed those usually required for obstetric pain because of increased tolerance of pain medications used for pain crises. Indications for cesarean section are obstetric and the same as for patients without sickle cell disease.
|
| Narcotics (IV) | Nonnarcotic Adjuncts (First-Second Trimester) |
|---|---|
|
|
Chronic anemia associated with sickle cell disease is exacerbated by the usual dilution effect of pregnancy. Iron repletion does not correct the anemia and should be avoided because many patients are already iron overloaded from repeat transfusions. The use of prophylactic blood transfusions is not supported. Simple or exchange transfusions should be instituted for the same indications as for nonpregnant patients, including stroke, ocular events, severe acute chest syndrome, splenic sequestration, symptomatic aplastic crisis, and cerebrovascular accident ( Box 1 ).
- •
Toxemia
- •
Severe anemia (decrease to 30% less than the baseline or Hb ≤6.0 g/dL)
- •
Acute renal failure
- •
Septicemia/bacteremia
- •
Acute chest syndrome with hypoxia or other severe sickle cell complication
- •
Anticipated surgery
Abbreviation: Hb, hemoglobin.
Pregnant patients with suspected sickle cell trait should be confirmed by hemoglobin electrophoresis. These women are at increased risk of bacteruria that may lead to urinary tract infections or pyelonephritis; thus, even asymptomatic bacteruria should be treated.
Thalassemia
Thalassemias are a result of quantitative disorders of hemoglobin production resulting from decreased or imbalanced production of generally structurally normal globins. In β-thalassemia, a mutation in β-globin leads to decreased production and an excess of α-globins and the reverse for mutations in α-globins in α-thalassemias. These mutations result in membrane damage and red cell fragility, causing microcytosis with target cell morphology and a chronic hemolytic anemia with compensatory reticulocytosis and splenomegaly. α-Thalassemias occur more commonly in Asian, African, or Mediterranean populations. β-Thalassemias occur more commonly in Mediterranean and African populations.
In general, women with α-thalassemia or β-thalassemia trait tolerate pregnancy well. These patients have a mild baseline microcytic anemia and, despite increased iron absorption characteristic of thalassemia, iron deficiency can develop and iron studies may need to be checked during pregnancy. Patients with severe α-thalassemia and β-thalassemia intermedia have compromised fertility, and few pregnancies have been reported. Approximately 50% of pregnancies result in stillbirth, intrauterine growth restriction, and preterm delivery. In a small number of patients, term pregnancy was attained with the use of transfusion support with target hemoglobin of 10 gm/dl.
Thrombocytopenia
Thrombocytopenia affects 10% of pregnancies and can be isolated or part of a systemic disorder. The thrombocytopenia cause may be specific to or unrelated to pregnancy. Most cases are asymptomatic and detected incidentally by routine screening of blood counts during pregnancy. Manifestations include easy bruising, petechiae, epistaxis, and gingival bleeding. Significant hemorrhage is rare, even when counts decrease to less than 20,000/μL. The major causes of thrombocytopenia in pregnancy are presented in Table 5 . A 10% decrease in platelets can commonly be seen in pregnancy. For general management, most women can continue routine obstetric care. The mode of delivery for patients with thrombocytopenia should be determined by obstetric indications. No studies have been conducted to determine the optimal platelet count but observational data indicate that a platelet count of more than 50,000/μL to 75,000/μL is adequate for epidural anesthesia, vaginal delivery, and cesarean section. However, the treating team and the anesthesiologist should individualize each patient’s care.
- •
Gestational thrombocytopenia is a benign form of thrombocytopenia that represents the most common cause of thrombocytopenia in pregnant woman. Patients typically have platelet counts greater than 70,000/μL. It is not associated with any adverse outcomes for the mother or the fetus. The cause of the thrombocytopenia is not well understood but could be related to diminished platelet survival combined with the physiologic increase in plasma volume. Gestational thrombocytopenia is considered a diagnosis of exclusion and it is often difficult to differentiate from idiopathic thrombocytopenia (ITP).
- •
ITP is found in approximately 1 in 10,000 pregnancies and usually presents in the first trimester. There have been isolated cases of ITP that cause thrombocytopenia in the neonate. No correlation has been found between maternal and fetal/neonatal platelet counts. Most cases of severe fetal or neonatal thrombocytopenia are related to alloimmune thrombocytopenia (discussed later). There is no evidence to support the need for intrapartum fetal platelet counts in patients with ITP. ITP in pregnancy is generally treated with either corticosteroids or intravenous immunoglobulin (IVIG) for similar indications as in nonpregnant patients, with an attempt to keep platelet counts at more than 50,000 to 70,000/μL near term. For refractory patients, a combination of corticosteroids and IVIG is recommended. Splenectomy can be considered but should be reserved for the second trimester. Rituximab, a pregnancy class C medication, has been used successfully in a limited number of pregnant women, including 1 case of ITP that was associated with asymptomatic neonatal B-lymphocyte suppression. The thrombopoietic agents romiplostim and eltrombopag are pregnancy class C medications, and there are no data to recommend use in pregnancy.
- •
Preeclampsia affects 6% of first pregnancies, and about 50% of these patients have thrombocytopenia. The pathophysiology of the thrombocytopenia is not well understood but accelerated platelet consumption is thought to contribute.
- •
HELLP (hemolysis, increased liver function tests, and low platelets) syndrome, which affects between 0.1% and 0.89% of pregnancies, shares many clinical features with preeclampsia and is often considered a variant of severe preeclampsia. HELLP has a higher rate of maternal and fetal mortality than preeclampsia, making correct diagnosis and treatment imperative. Patients with HELLP typically have a microangiopathic hemolytic anemia with schistocytes seen on peripheral blood smear, an increased lactate dehydrogenase (LDH), serum levels of aspartate aminotransferase greater than 70 U/L, and platelet counts less than 100,000/μL. HELLP is often difficult to differentiate from thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS). Treatment of preeclampsia and HELLP consists of stabilization of the mother followed by expeditious delivery, which usually results in resolution of the disorder within 3 to 4 days. However, both syndromes, especially HELLP, occasionally worsen or develop in the postpartum period. There are limited data from several small, randomized studies of the use of corticosteroids in the prenatal or postnatal setting. Corticosteroids for HELLP are thought to hasten the resolution of thrombocytopenia and laboratory abnormalities when used 5 to 7 days after delivery for worsening thrombocytopenia or other signs of clinical decline. However, clear efficacy rather than just correction of laboratory abnormalities has not been shown.
- •
TTP risk increases during pregnancy. Pregnant women comprise 10% to 20% of patients with TTP. TTP typically develops in the second or third trimester. Treatment is plasma exchange. The risk of HUS also increases with pregnancy and most cases develop 3 to 4 weeks after birth. Atypical HUS with renal failure as the predominant manifestation is the most common. The prognosis of postpartum HUS is poor, with more than 25% of patients with persistent renal failure. Plasma exchange is recommended despite low response rates because of the difficulty differentiating HUS from TTP.
- •
Disseminated intravascular coagulation (DIC) may accompany preeclampsia and may also result from retained fetal products, sepsis, placental abruption, or amniotic fluid embolization. In general, the thrombocytopenia is milder and the degree of microangiopathic hemolysis is less than with TTP, HUS, or HELLP. DIC in pregnancy can be abrupt, severe, and fatal if not addressed appropriately. Therapy is aimed at treating the underlying condition that precipitated the DIC. The British Committee for Standards in Haematology has published guidelines for the management of DIC. Transfusion support should be based on the presence of bleeding from low platelets or a prolonged prothrombin time. Cryoprecipitate may be used for severe hypofibrinogenemia (fibrinogen <100 g/dL) that persists after plasma therapy. Low doses of heparin may be indicated for patients with significant thrombosis.
- •
Acute fatty liver of pregnancy, another pregnancy-specific cause of thrombocytopenia, usually occurs during the third trimester in primaparas and twin gestations. Typical symptoms are usually nausea, vomiting, right upper quadrant pain, anorexia, jaundice, and increased liver enzymes consistent with cholestasis. Most patients have concurrent DIC. Diabetes insipidus and hypoglycemia are present in more than half of cases. Bleeding is common, caused by coagulopathy resulting from diminished hepatic synthesis of coagulation factors, as well as DIC and acquired antithrombin deficiency.
- •
Additional causes of thrombocytopenia include pseudothrombocytopenia (caused by platelet aggregation induced by ethylenediamine tetraacetic acid); hypersplenism; congenital platelet disorders such as Bernard-Soulier syndrome, May-Hegglin anomaly, gray platelet syndrome, and Glanzmann thrombasthenia; bone marrow disease; drug-induced, human immunodeficiency virus, and other autoimmune thrombocytopenias; congenital thrombocytopenias; and type 2B Von Willebrand Disease (VWD; discussed later).
| Incidence (%) | Timing of Onset | Degree of Thrombocytopenia | Microangiopathic Chemolytic Anemia | Hypertension | Coagulopathy | Liver Disease | Renal Disease | CNS Disease | |
|---|---|---|---|---|---|---|---|---|---|
| ITP | 3−4 | Most common in first trimester, anytime | Mild to severe | None | None | None | None | None | None |
| Gestational/incidental thrombocytopenia | 75−80 | Second−third trimester | Mild | None | None | None | None | None | None |
| Preeclampsia | 15−20 | Late second to third trimester | Mild to moderate | Mild | Moderate to severe | None to mild | None | Proteinuria | Seizures with preeclampsia |
| HELPP | — | Late second to third trimester | Moderate to severe | Moderate to severe | None to severe | Absent to mild | Moderate to severe | None to moderate | None to moderate |
| DIC | Rare | Anytime | Moderate to severe | Mild to moderate | None | Mild to severe | Variable | Variable | None |
| AFLP | Rare | Third trimester | Mild | Mild | None to mild | Severe | Severe | None to mild | None to mild |
| TTP | Rare | Second to third trimester | Severe | Moderate to severe | None | None | None | None to moderate | None to severe |
| HUS | Rare | After birth | Moderate to severe | Moderate to severe | None to mild | None | None | Moderate to severe | None to mild |
Thrombocytopenia
Thrombocytopenia affects 10% of pregnancies and can be isolated or part of a systemic disorder. The thrombocytopenia cause may be specific to or unrelated to pregnancy. Most cases are asymptomatic and detected incidentally by routine screening of blood counts during pregnancy. Manifestations include easy bruising, petechiae, epistaxis, and gingival bleeding. Significant hemorrhage is rare, even when counts decrease to less than 20,000/μL. The major causes of thrombocytopenia in pregnancy are presented in Table 5 . A 10% decrease in platelets can commonly be seen in pregnancy. For general management, most women can continue routine obstetric care. The mode of delivery for patients with thrombocytopenia should be determined by obstetric indications. No studies have been conducted to determine the optimal platelet count but observational data indicate that a platelet count of more than 50,000/μL to 75,000/μL is adequate for epidural anesthesia, vaginal delivery, and cesarean section. However, the treating team and the anesthesiologist should individualize each patient’s care.
- •
Gestational thrombocytopenia is a benign form of thrombocytopenia that represents the most common cause of thrombocytopenia in pregnant woman. Patients typically have platelet counts greater than 70,000/μL. It is not associated with any adverse outcomes for the mother or the fetus. The cause of the thrombocytopenia is not well understood but could be related to diminished platelet survival combined with the physiologic increase in plasma volume. Gestational thrombocytopenia is considered a diagnosis of exclusion and it is often difficult to differentiate from idiopathic thrombocytopenia (ITP).
- •
ITP is found in approximately 1 in 10,000 pregnancies and usually presents in the first trimester. There have been isolated cases of ITP that cause thrombocytopenia in the neonate. No correlation has been found between maternal and fetal/neonatal platelet counts. Most cases of severe fetal or neonatal thrombocytopenia are related to alloimmune thrombocytopenia (discussed later). There is no evidence to support the need for intrapartum fetal platelet counts in patients with ITP. ITP in pregnancy is generally treated with either corticosteroids or intravenous immunoglobulin (IVIG) for similar indications as in nonpregnant patients, with an attempt to keep platelet counts at more than 50,000 to 70,000/μL near term. For refractory patients, a combination of corticosteroids and IVIG is recommended. Splenectomy can be considered but should be reserved for the second trimester. Rituximab, a pregnancy class C medication, has been used successfully in a limited number of pregnant women, including 1 case of ITP that was associated with asymptomatic neonatal B-lymphocyte suppression. The thrombopoietic agents romiplostim and eltrombopag are pregnancy class C medications, and there are no data to recommend use in pregnancy.
- •
Preeclampsia affects 6% of first pregnancies, and about 50% of these patients have thrombocytopenia. The pathophysiology of the thrombocytopenia is not well understood but accelerated platelet consumption is thought to contribute.
- •
HELLP (hemolysis, increased liver function tests, and low platelets) syndrome, which affects between 0.1% and 0.89% of pregnancies, shares many clinical features with preeclampsia and is often considered a variant of severe preeclampsia. HELLP has a higher rate of maternal and fetal mortality than preeclampsia, making correct diagnosis and treatment imperative. Patients with HELLP typically have a microangiopathic hemolytic anemia with schistocytes seen on peripheral blood smear, an increased lactate dehydrogenase (LDH), serum levels of aspartate aminotransferase greater than 70 U/L, and platelet counts less than 100,000/μL. HELLP is often difficult to differentiate from thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS). Treatment of preeclampsia and HELLP consists of stabilization of the mother followed by expeditious delivery, which usually results in resolution of the disorder within 3 to 4 days. However, both syndromes, especially HELLP, occasionally worsen or develop in the postpartum period. There are limited data from several small, randomized studies of the use of corticosteroids in the prenatal or postnatal setting. Corticosteroids for HELLP are thought to hasten the resolution of thrombocytopenia and laboratory abnormalities when used 5 to 7 days after delivery for worsening thrombocytopenia or other signs of clinical decline. However, clear efficacy rather than just correction of laboratory abnormalities has not been shown.
- •
TTP risk increases during pregnancy. Pregnant women comprise 10% to 20% of patients with TTP. TTP typically develops in the second or third trimester. Treatment is plasma exchange. The risk of HUS also increases with pregnancy and most cases develop 3 to 4 weeks after birth. Atypical HUS with renal failure as the predominant manifestation is the most common. The prognosis of postpartum HUS is poor, with more than 25% of patients with persistent renal failure. Plasma exchange is recommended despite low response rates because of the difficulty differentiating HUS from TTP.
- •
Disseminated intravascular coagulation (DIC) may accompany preeclampsia and may also result from retained fetal products, sepsis, placental abruption, or amniotic fluid embolization. In general, the thrombocytopenia is milder and the degree of microangiopathic hemolysis is less than with TTP, HUS, or HELLP. DIC in pregnancy can be abrupt, severe, and fatal if not addressed appropriately. Therapy is aimed at treating the underlying condition that precipitated the DIC. The British Committee for Standards in Haematology has published guidelines for the management of DIC. Transfusion support should be based on the presence of bleeding from low platelets or a prolonged prothrombin time. Cryoprecipitate may be used for severe hypofibrinogenemia (fibrinogen <100 g/dL) that persists after plasma therapy. Low doses of heparin may be indicated for patients with significant thrombosis.
- •
Acute fatty liver of pregnancy, another pregnancy-specific cause of thrombocytopenia, usually occurs during the third trimester in primaparas and twin gestations. Typical symptoms are usually nausea, vomiting, right upper quadrant pain, anorexia, jaundice, and increased liver enzymes consistent with cholestasis. Most patients have concurrent DIC. Diabetes insipidus and hypoglycemia are present in more than half of cases. Bleeding is common, caused by coagulopathy resulting from diminished hepatic synthesis of coagulation factors, as well as DIC and acquired antithrombin deficiency.
- •
Additional causes of thrombocytopenia include pseudothrombocytopenia (caused by platelet aggregation induced by ethylenediamine tetraacetic acid); hypersplenism; congenital platelet disorders such as Bernard-Soulier syndrome, May-Hegglin anomaly, gray platelet syndrome, and Glanzmann thrombasthenia; bone marrow disease; drug-induced, human immunodeficiency virus, and other autoimmune thrombocytopenias; congenital thrombocytopenias; and type 2B Von Willebrand Disease (VWD; discussed later).