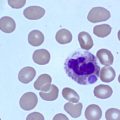
Primary polycythemias are the result of intrinsic abnormalities of the hematopoietic progenitors that lead to constitutive overproduction of red cells accompanied by low erythropoietin (EPO) levels. Secondary polycythemias are caused by conditions resulting in increased EPO production. Polycythemia vera (PV) is a primary polycythemia, and is a chronic clonal progressive myeloproliferative neoplasm. A single recurrent point mutation in the pseudokinase domain of JAK2 molecule (JAK2 V617F ) is present in >95% of patients with PV. The goal of therapy in PV is to normalize blood counts to minimize the risk of thrombotic events.
The term polycythemia comes from the Greek, meaning too many cells in the blood, and refers to an increase in the red cell mass; it is frequently used interchangeably with the term erythrocytosis. Polycythemia may have several causes, and can be classified as relative or absolute. Relative polycythemia occurs when the patient has a modest increase of the hematocrit level without an increased red cell mass caused by contraction of the plasma volume. Absolute polycythemia is accompanied by an increase in the circulating red cell mass. Red cell production can be influenced by numerous factors including nutrients, growth factors, numbers, and function of marrow progenitor and precursor cells, as well as cellular receptors and transcription factors. The hematopoietic growth factor erythropoietin (EPO) is considered to be the physiologic regulator of the terminal phases of erythropoiesis. Alterations in its production are followed by adjustments in the rate of formation of red blood cells.
Hematocrit values more than 52% in men and more than 48% in women are abnormal and require further investigation into their causes. Polycythemic states can also be divided into primary or secondary polycythemias ( Box 1 ). Primary polycythemias are the result of intrinsic abnormalities of the hematopoietic progenitors and stem cells that lead to constitutive overproduction of red cells accompanied by low EPO levels. Secondary polycythemias can be a result of several conditions causing increased EPO production, which acts on normal progenitors to increase the red cell production. These conditions can usually be distinguished by in vitro assays of erythroid progenitor cells, measurement of serum EPO levels, and detection of somatic JAK2 mutations. In a small number of patients, the cause of eythrocytosis cannot be determined, and these patients are classified as having idiopathic erythrocytosis.
Secondary polycythemias
Appropriately increased EPO
Cyanotic heart disease
Pulmonary disease
Obstructive sleep apnea
Pickwickian syndrome
Smokers’ polycythemia, hookah polycythemia
High altitude
Inappropriately Increased EPO
Renal cell carcinoma
Hepatocellular carcinoma
Uterine fibroma
Hemangioblastoma
Renal lesions (cysts, hydronephrosis)
Following renal transplantation
Drug induced polycythemia
Congenital polycythemias
Chuvash polycythemia and abnormalities of O 2 sensing
Methemoglobinemia
2,3-bisphosphoglycerate (BPG) deficiency
Primary polycythemias
Polycythemia vera (PV)
Primary familial and congenital polycythemias (activating mutations of EPO receptor [EPOR] and mutations yet to be determined)
Regulation of erythropoiesis
The interaction of EPO with EPOR on erythroid progenitor cells leads to its homodimerization, which results in initiation of cell division, differentiation, and prevention of apoptosis of erythroid progenitors and precursors. The cytoplasmic portion of the EPOR contains a positive regulatory domain that interacts with JAK2. On EPO binding, JAK2 phosphorylates itself, EPOR, and other cytoplasmic molecules including STAT5. The JAK2/STAT5 signaling pathway plays an important role in EPOR-mediated regulation of erythropoiesis.
Under normal conditions, EPO production is regulated by the decreased oxygen content of hemoglobin within red cells, resulting in decreased oxygen delivery to the tissues. In response to chronic hypoxia, multiple compensatory mechanisms take place over several days in the kidneys, which is the major site of EPO production. Hypoxia results in the production of hypoxia-inducible factor (HIF) 1, the major factor responsible for transcriptional activation of the EPO gene. HIF-1 is a heterodimeric protein consisting of HIF-1α and HIF-1β. The levels of HIF-1α increase exponentially as the oxygen concentration declines. HIF-1 facilitates body oxygen delivery and responds to oxygen deprivation by regulating gene expression involved in cellular energy metabolism, pH regulation, apoptosis, erythropoiesis and iron metabolism, cell proliferation, and cell-cell and cell-matrix interactions.
HIF-1α is constitutively expressed, but it is barely detectable in normal conditions caused by/because of rapid degradation by the ubiquitin-proteosome pathway. In conditions of hypoxia HIF-1α is rapidly accumulated because of the lack of degradation. This mechanism results in instantaneous response to hypoxia. The targeting and subsequent ubiquitination of HIF-1α requires the von Hippel-Lindau protein (VHL), oxygen, and 3 different iron-requiring proline hydroxylase (PHD) enzymes. The proline hydroxylation is required for binding of HIF-1α to VHL. VHL protein is part of a multiprotein ubiquitin ligase capable of ubiquitinating HIF-1α subunits and targeting them for destruction by the proteasome. As oxygen levels decrease, hydroxylation of HIF-1α decreases, and it can no longer bind VHL, thus avoiding degradation. HIF-1α dimerizes with HIF-1β and activates transcription of target genes. The activity of PHDs depends on the availability of oxygen, which makes these enzymes oxygen sensors.
Another protein involved in this pathway is HIF-2α which dimerizes with HIF-1β in hypoxic conditions and activates the transcription of a set of target genes that overlap with the genes targeted by HIF-1α/HIF-1β heterodimers. HIF-1α is expressed by all cell types, whereas HIF-2α is expressed only in specific cell types including vascular endothelial cells, hepatocytes, cardiomyocytes, renal interstitial cells, and astrocytes.
Relative polycythemias
Patients with moderately increased hematocrit levels that are not necessarily accompanied by an increase in red cell mass are often erroneously assumed to be polycythemic. Relative polycythemia is a term used to describe an increase of the hematocrit level due either to an acute transient state of hemoconcentration associated with intravascular volume depletion or a chronic, sustained, relative polycythemia caused by contraction of the plasma volume. Transient polycythemia may be a result of acute depletion of the plasma volume as a result of protracted vomiting or diarrhea, plasma loss from external burns, or insensible fluid loss caused by fever or diabetic ketoacidosis.
Another example of relative polycythemia is Gaisböck syndrome, which is a benign condition observed mainly in obese, hypertensive, middle-aged, male smokers. These individuals can have a chronic modest increase in their hematocrit levels without increase in their red cell mass. This observation has been attributed to reduced venous compliance.
Relative polycythemias
Patients with moderately increased hematocrit levels that are not necessarily accompanied by an increase in red cell mass are often erroneously assumed to be polycythemic. Relative polycythemia is a term used to describe an increase of the hematocrit level due either to an acute transient state of hemoconcentration associated with intravascular volume depletion or a chronic, sustained, relative polycythemia caused by contraction of the plasma volume. Transient polycythemia may be a result of acute depletion of the plasma volume as a result of protracted vomiting or diarrhea, plasma loss from external burns, or insensible fluid loss caused by fever or diabetic ketoacidosis.
Another example of relative polycythemia is Gaisböck syndrome, which is a benign condition observed mainly in obese, hypertensive, middle-aged, male smokers. These individuals can have a chronic modest increase in their hematocrit levels without increase in their red cell mass. This observation has been attributed to reduced venous compliance.
Absolute polycythemias
Primary Familial and Congenital Polycythemias
Primary familial and congenital polycythemias (PFCP) is an autosomal dominant disorder. Unlike patients with PV, patients with PFCP lack splenomegaly and do not progress to myelofibrosis and acute leukemia. They often present with headaches, epistaxis, exertional dyspnea, and dizziness. An increased risk of cardiovascular events and resulting premature morbidity and mortality have been reported in these patients. Although clinical symptoms are resolved with reduction in red cell mass via phlebotomies, the increased risk of cardiovascular events remains. Characteristic laboratory findings are increased hematocrit without associated leukocytosis or thrombocytosis, lack of activating JAK2 mutation, low serum EPO levels, normal hemoglobin-oxygen dissociation curves, and in vitro hypersensitivity of erythroid progenitors to EPO. Between 10% and 20% of patients with PFCP have mutations in EPOR. Additional disease-causing genes and their mutations have yet to be identified.
Secondary Polycythemias
Secondary polycythemias can be either congenital or acquired (see Box 1 ). Conditions leading to hypoxia, such as high altitude, cyanotic heart disease, or chronic lung disease, may result in physiologic polycythemia mediated by increased levels of EPO. There are marked variations in EPO levels and the subsequent erythroid response in the face of chronic hypoxia, suggesting that some of these factors may be genetically determined.
Acquired secondary polycythemias
Polycythemias associated with cyanotic heart disease and pulmonary disease
Patients with cyanotic heart disease and pulmonary disease frequently suffer from arterial hypoxemia leading to increased production of EPO and polycythemia. Excessive EPO production occurs when the Pa o 2 is sustained at less than 67 mm Hg as a result of severely impaired pulmonary function. Moderate increases of the hematocrit have been estimated to occur in 20% of patients with chronic obstructive pulmonary disease (COPD). Polycythemia in this setting can contribute to pulmonary hypertension, pulmonary endothelial cell dysfunction, reduced cerebral blood flow, and increased risk of venous thromboembolic disease. Increased oxygen-carrying capacity may improve oxygen delivery; however, it is not obvious at what hematocrit level the resultant increase in blood viscosity impairs blood flow to the tissues, leading to a reduction in oxygen uptake.
The treatment of hyperviscosity secondary to erythrocytosis in cyanotic heart disease with prophylactic phlebotomy was once a core therapeutic intervention, but is not considered so today. Currently, experts recommend that phlebotomy should be restricted to individuals with symptoms of extreme erythrocytosis (hematocrit >65%) and before surgery to improve hemostasis.
Obstructive sleep apnea–induced polycythemia
Obstructive sleep apnea syndrome is characterized by repetitive episodes of partial or complete obstruction of airflow during sleep. Although the evidence is largely anecdotal, secondary polycythemia is a widely recognized complication of long-standing sleep apnea, being found in 5% to 10% of those with nocturnal apnea and hypopnea. Similarly, 25% of those with unexplained polycythemia are subsequently found to have sleep apnea.
Pickwickian syndrome and polycythemia
Pickwickian syndrome, or obesity-hypoventilation syndrome, seen in morbidly obese individuals, is characterized by chronic hypoxemia and hypercapnia caused by alveolar hypoventilation, with a resultant increase in EPO production, polycythemia, and cor pulmonale. The 3 principal causes are the high cost of the work of respiration in morbidly obese individuals, dysfunction of the respiratory centers, and repeated episodes of nocturnal obstructive apnea.
Polycythemia caused by high altitude
Hypoxic conditions associated with high altitude result in adoptive hyperventilation, alkalosis, and shifting of the O 2 dissociation curve to the left, which leads to impaired oxygen release to the tissues and tissue hypoxia. This hypoxia causes markedly increased EPO production leading to erythrocytosis. Residents of the Andes Mountains who live 4200 m above sea level frequently have 30% higher hematocrit levels than individuals living at sea level. There is evidence of individual variation in response to hypoxia seen both in cyanotic heart and pulmonary disease and in exposure to high altitude, with not all affected individuals generating an appropriate increase in red cell mass. This individual variation suggests that there are genetic determinants underlying the erythropoietic response to hypoxia.
Smokers’ polycythemia or carbon monoxide–induced polycythemia
Smoking is the most common cause of secondary polycythemia. Those affected have a carboxyhemoglobin-induced increase in red cell mass and/or decrease in plasma volume, either of which is reversible with smoking cessation. Excessive carbon monoxide exposure can also be attributed to exposure to industrial emissions and automobile exhaust. Individuals smoking even 1 pack of cigarettes a day frequently have increased hematocrit levels. These patients characteristically have normal blood gases and increase of carboxyhemoglobin levels. The increase of the hematocrit level is reversed with cessation of smoking. Increased hematocrit levels have been observed in 3% to 5% of heavy smokers. Although these patients do develop thrombotic complications, the rate of thromboembolic events is lower compared with the rate of thromboembolic events in patients with PV. Recently polycythemia was also reported with hookah use, which exposes the user to large amounts of carbon monoxide, resulting in erythrocytosis.
Post–renal transplantation erythrocytosis
Post–renal transplantation erythrocytosis (PTE) is defined as a persistently increased hematocrit level greater than 51% after renal transplantation without an increase of the white blood cell count or platelet count. It is found in approximately 10% to 15% of renal allograft recipients. PTE is usually seen 8 to 24 months after a successful renal transplantation. At higher hematocrit levels (usually 60%), thrombotic complications may develop.
Approximately 60% of patients with PTE experience malaise, headache, plethora, lethargy, and dizziness. In addition, from 10% to 20% develop thromboembolic complications involving either arteries or veins. Retention of the native kidney is essential for the development of PTE in most cases. Although the transplanted kidney produces EPO under normal regulatory mechanisms, the native kidney overproduces EPO in spite of the development of erythrocytosis. The PTE frequently resolves with removal of the native kidney. Plasma EPO levels are higher (10-fold) in patients with PTE compared with nonerythrocytotic renal transplant recipients. Treatment of patients with PTE includes intermittent phlebotomy or administration of drugs. The angiotensin-converting enzyme inhibitor enalapril suppresses the renin-angiotensin pathway and virtually eliminates the need for therapeutic phlebotomy in these patients. Therapy is indicated at hematocrit levels of more than 55% with the hope of maintaining hematocrit levels at less than 50% to reduce the risk of thrombosis.
Polycythemia associated with renal and liver disorders and neoplasms
Polycythemia has been reported to be associated with renal cell carcinoma, Wilms tumor, polycystic kidney disease, renal artery stenosis, hydronephrosis, paragangliomas, and pituitary adenomas. Renal tumors account for one-third of cases of tumor-associated polycythemia. Tumor tissues produce excessive amounts of EPO and tumor resection results in correction of erythrocytosis. Polycythemia has also been described in association with hepatocellular carcinomas in 2% to 10% of patients. Cerebellar hemangioblastomas and large uterine fibromas are also associated with polycythemia.
Drug-induced erythrocytosis and polycythemia in endocrine disorders
The long-term administration of EPO, corticosteroids, or androgens can be associated with reversible erythrocytosis. Polycythemia can also be associated with Cushing syndrome, acromegaly, and primary aldosteronism. It is also observed in older men receiving long-term androgen replacement therapy and in significant numbers of athletes taking anabolic steroids.
Androgens have been shown to stimulate EPO production and to directly affect erythroid progenitor cells. Blood doping refers to the use of EPO to increase red cell mass in athletes, thus increasing oxygen delivery in the hope of increasing endurance.
Congenital secondary polycythemias
High-oxygen-affinity hemoglobins and bisphosphoglycerate (BPG) deficiency
There are more than a 100 mutations in the hemoglobin genes leading to increased affinity for oxygen and thus decreased oxygen delivery, resulting in compensatory erythrocytosis ( Table 1 ). These mutations are usually well tolerated in younger patients, but older patients may develop thrombotic complications. These high-affinity hemoglobin mutations are transmitted in autosomal dominant fashion. Phlebotomy therapy for these patients has not been shown to have any clinical benefit and may be detrimental with decreased exercise tolerance and anaerobic threshold.
| Specific Conditions | Method of Transmission and Relevant Clinical Presentations | Specific Genes Involved |
|---|---|---|
| Alterations in hemoglobin oxygen affinity | ||
| High O 2 affinity hemoglobin mutants | Autosomal dominant Patients usually asymptomatic | Mutations of both a and b globin genes |
| 2,3 BPG deficiency | Unclear | Deficiency of red cell enzyme bisphosphoglyceromutase |
| Congenital methemoglobinemia | Autosomal recessive Most patients are asymptomatic. May have blue color of the skin and mucous membranes (cyanotic) | Deficiency of cytochrome b5 reductase Hemoglobin M disease |
| Alterations in hypoxia sensing | ||
| Chuvash polycythemia mutations in VHL gene | Autosomal recessive High hemoglobin levels, increased EPO levels, varicose veins, vertebral hemangiomas, hypotension. High morbidity and mortality from thrombotic and hemorrhagic complications | Mutations in VHL gene |
| Proline hydroxylase 2 (PHD2) gene mutation | Autosomal dominant Increased EPO levels | PHD2 gene mutations |
| Hypoxia-inducible factor 2 gene mutation | Autosomal dominant | HIF-2 gene mutations |
| Polycythemias with undefined defects | ||
| PFCP | Autosomal dominant | Activating mutations in EPOR only in 10%–20% of patients |
A rare cause of congenital polycythemia is 2,3-BPG deficiency. It is synthesized in the red blood cells and acts by reducing hemoglobin’s affinity for oxygen. Its absence leads to higher oxygen affinity and results in hypoxic stimulus and erythrocytosis.
HIF pathway mutations
Mutations in several proteins in the HIF pathway have been shown to lead to increased EPO production and erythrocytosis (see Table 1 ). Chuvash polycythemia (CP) is one of the best-known examples. CP is an autosomal recessive disorder associated with germline mutations in VHL, and is endemic in the Chuvash population of the Russian republic. A homozygous, missense mutation in the VHL gene (VHL598C→T) has been identified in patients with CP. This disorder is characterized by high hemoglobin levels, usually more than 20 g/dL, increased EPO levels, varicose veins, vertebral hemangiomas, and low blood pressure. The defective VHL gene product is not capable of promoting ubiquitin-mediated degradation of HIF-1α and HIF-2α, leading to their increased levels and thus increased production of EPO. Several similar types of mutations have been described in the VHL gene and have been found in a variety of other ethnic groups. CP is associated with high mortality caused by thrombotic and hemorrhagic vascular complications.
A mutation in the proline hydroxylase gene PHD2 (P317R) has been described in a family with autosomal dominant erythrocytosis (see Table 1 ). The mutated protein fails to cause degradation of HIF-1α and results in increased EPO levels and erythrocytosis.
A series of HIF-2α gain-of-function mutations have also been reported to lead to familial erythrocytosis. These patients present with congenital erythrocytosis and with increased or inappropriately normal EPO levels.
Polycythemia vera (PV)
Introduction and epidemiology
PV is a chronic, clonal, progressive, myeloproliferative neoplasm (MPN) characterized by absolute increase in red cell mass, as well as splenomegaly, leukocytosis, and thrombocytosis. PV is the most common primary polycythemia, with reported incidences of 2.8 per 100,000 persons per year. The prevalence of PV has been reported to be higher among Ashkenazi Jews and lower among African Americans. Extremely low occurrence rates have been reported from Japan. These findings suggest the importance of genetic factors in the pathogenesis of this disease. This importance is further emphasized by reports of multiple cases of MPN including PV in multiple generations of several families. In a large population study reported from Sweden, it was estimated that the first-degree relatives of patients with MPN have a 5-fold to 7-fold increased risk of developing an MPN. Higher incidence of PV has been reported in individuals exposed to the atomic bomb explosion in Japan, which is a notable exception to the overall low rate of PV in Japan. Increased incidence of PV has also been reported in workers in petroleum refineries and chemical plants. Environmental triggers have been further implicated after a description of a cluster of patients with JAK2 V617F -positive PV in an area of eastern Pennsylvania that contained numerous sources of hazardous materials. Slightly more men than women develop PV, with a male/female ratio of approximately 1.2:1. The average age at diagnosis is 60 years; in several large studies, 5% of patients were younger than 40 years of age. PV has been reported to present in childhood in a small number of patients.
Pathobiology
The expanded red cell mass in PV is a result of a 2-fold to 3-fold increase in the production of red cells by a hyperplastic marrow. Granulocyte and platelet production is also increased in this disorder. Various methods have been used since the mid-1970s to show that PV is a result of clonal proliferation of neoplastic hematopoietic stem cells, and is not a consequence of excessive proliferation of normal hematopoietic stem cells.
From the knowledge that red cell production in PV is not associated with excessive EPO production, it was hypothesized that erythroid progenitor cells in this disorder are no longer subject to physiologic regulators. PV bone marrow can form erythroid colonies termed endogenous colonies in vitro in the absence of exogenous EPO, whereas normal bone marrow is incapable of forming such colonies without the addition of EPO.
JAK2 V617F mutation and other JAK2 mutations in Polycythemia vera (PV)
The genetic defect associated with PV was a focus of intense research by numerous groups. In 2005, JAK2 from hematopoietic cells of patients with PV was directly sequenced by Vainchenker and colleagues in France and a single recurrent point mutation was discovered. A guanine-to-thymine mutation was observed that resulted in a substitution of valine to phenylalanine at codon 617 within the pseudokinase domain (JH2) of Janus kinase 2 (JAK2 V617F ). These findings were rapidly confirmed by several other groups. Analysis of germline DNA showed that JAK2 V617F is an acquired somatic mutation present exclusively in hematopoietic cells. Using quantitative polymerase chain reaction, patients can be classed as having a low allele burden of JAK2 V617F (<50%) or a high allele burden of JAK2 V617F (>50%). A subset of patients with PV is homozygous for JAK2 V617F , which was shown to be the result of mitotic recombination and duplication of the mutant allele. The occurrence of this mitotic recombination event has been observed during the clinical course of individual patients, with heterozygous patients becoming homozygous over the course of their disease.
The mutational frequency of JAK2 V617F is more than 95% in patients with PV, and 50% to 60% in patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF). ET is usually associated with a low allele burden of JAK2 V617F .
Other mutations in JAK2 have also been shown to be associated with erythrocytosis. Several gain-of-function mutations in exon 12 of the JAK2 gene, adjacent to the pseudokinase domain, have been identified in 2.5% to 3.4% of patients with PV, and in 30% of patients who have JAK2 V617F -negative PV. JAK2 exon 12 mutations have not been reported in patients with ET or PMF. Patients with exon 12 mutations can develop thrombotic events and can also evolve into post-PV myelofibrosis (MF) and acute leukemia.
Clinical manifestations of Polycythemia vera (PV)
The principal clinical manifestations of PV can be attributed to the excessive production of cells of myeloid lineage affected by the malignant clone. Symptomatic patients can present with a myriad of generalized symptoms, such as weakness, dizziness, pruritus, headaches, visual disturbances, excessive sweating, abdominal discomfort, weight loss, and thrombotic or hemorrhagic episodes. Thrombosis is a frequent presenting event with two-thirds of these episodes occurring either at presentation or before diagnosis. The principal findings on physical examination of a patient with PV include ruddy cyanosis, conjunctival plethora, hepatomegaly, splenomegaly, and hypertension. Untreated patients are at a particularly high risk for thrombotic complications. Arterial thrombotic events account for two-thirds of these episodes, with transient ischemic attacks, ischemic strokes, and myocardial infarctions being the most frequent complications. The cumulative rate of thrombosis ranges from 2.5% to 5% per patient per year. It is usual for these patients to develop thrombosis in unusual sites, such as splanchnic veins including hepatic, splenic, or mesenteric vessels, or in a cerebral sinus. Budd-Chiari syndrome is one of the most serious thrombotic complications of PV, and results from hepatic venous or inferior vena cava thrombosis and can culminate in the development of liver cirrhosis. Patients can present with portal or hepatic vein thrombosis and have normal values of hemoglobin and hematocrit. They often have leukocytosis, increased platelet count, and splenomegaly. Screening for JAK2 V617F mutation should be performed in these patients. In patients with splanchnic vein thrombosis who are JAK2 V617F negative, a bone marrow biopsy should be performed to assess for a JAK2 V617F -negative MPN. In these patients, gastrointestinal bleeding or increased plasma volume caused by splenomegaly may be responsible for normal hematocrit.
PV can often present with symptoms of peripheral vascular disease, such as intense redness or cyanosis of the digits, classic erythromelalgia, digital ischemia with normal pulses, or thrombophlebitis. Erythromelalgia is described as a sensation of burning pain and increased temperature in the digits. PV is the most common cause of erythromelalgia, and is one of the few disorders in which digital ischemia exists in the presence of palpable pulses. Foot pain at rest is another distressing symptom of PV. The pain is most severe at night and is dull in nature. These symptoms have been shown to be a result of platelet aggregation and activation in vivo, which preferentially occurs in arterioles. Phlebotomy alone does not relieve erythromelalgia. These symptoms can be abolished by reducing the platelet counts to normal levels. Symptoms also improve with antiplatelet therapy.
Neurologic abnormalities can occur in up to 60% to 80% of untreated or poorly controlled patients with PV and include transient ischemic attacks, cerebral ischemia, cerebral hemorrhage, fluctuating dementia, and confusion. In addition, symptoms of dizziness, visual disturbances, tinnitus, paresthesias, and headaches are commonly reported. These symptoms are attributed to increased blood viscosity and reduced cerebral blood flow caused by erythrocytosis.
Patients with PV have greater than expected incidence of pulmonary hypertension. Possible mechanisms include extramedullary hematopoiesis in lung parenchyma, direct obstruction of pulmonary vessels by circulating megakaryocytes, smooth muscle hyperplasia induced by release of platelet-derived growth factor from activated platelets, and recurrent thrombotic events.
Up to 30% to 40% of patients with PV experience some type of hemorrhagic event, ranging from inconsequential epistaxis or gingival bleeding to life-threatening gastrointestinal hemorrhages.
Patients with PV undergoing a surgical procedure are at high risk of developing postoperative complications. Complications can be caused by thrombosis or hemorrhage. A retrospective study by an Italian group evaluated 311 surgical interventions in 105 patients with PV and 150 with ET: 24 arterial or venous thrombosis (7.7%), 23 major hemorrhages (7.3%), and 5 surgery-related deaths (1.6%) were observed within 3 months of the procedure. Patients with PV with uncontrolled erythrocytosis before surgery have the highest rate of complications after surgery. These complications can be greatly reduced by achieving normalization of blood counts before surgery. The risk seems to be even lower in patients who achieved long-term blood count control before surgery.
Generalized pruritus occurs in 40% of patients. Water contact such as during a shower or bath can induce attacks of intolerable pruritus. The degree of pruritus has no correlation with severity of disease and 20% of patients continue to experience severe pruritus despite adequate control of their blood counts.
There have been reports of patients with PV suffering nonhematologic complications of iron deficiency. However, there is no convincing evidence that undue fatigue caused by iron deficiency occurs in patients with normal or increased hematocrit levels. These patients do not experience esophageal changes or dysphagia that is known to be associated with chronic iron deficiency.
The risk of development of post-PV MF increases with prolonged duration of disease and should be considered the natural evolution of disease. For patients with disease duration greater than 10 years, the hazard ratio was reported to be 15.24 (95% confidence interval 4.22–55.06, P <.0001). The median interval between diagnosis of PV and development of post-PV MF is 13 years. Post-PV MF is characterized by increasing splenomegaly, leukoerythroblastic characteristics on peripheral blood smear review (tear drop morphology of red cells, nucleated red cells), development of extensive bone marrow fibrosis, and normal or decreased red cell mass. The patients can complain of fatigue, dizziness, and weight loss. Splenomegaly can lead to abdominal pain and early satiety. Patients with post-PV MF are virtually all JAK2 V617F positive and have high JAK2 V617F allele burden.
The median survival for patients with post-PV MF is 5.7 years and these patients are at high risk of developing acute leukemia. Eighteen percent of patients with post-PV MF undergo leukemic transformation after 3 years. Patients with hemoglobin of less than 10 g/dL, platelet count less than 100×10 9 /L, and white blood cell counts more than 30×10 9 /L have a poorer prognosis than patients who have fewer of these prognostic factors.
Differential diagnosis
The first step in establishing the cause of erythrocytosis is to confirm that it is absolute erythrocytosis. A hematocrit level greater than 60% on several occasions in men or greater than 55% in women is associated with an increased red cell mass in virtually every case. Documentation of an absolute increase in red cell mass by direct measurement is rarely required and this test is now available in few institutions.
Initially, it is important to differentiate PV from other causes of secondary erythrocytosis (see Box 1 ). Characteristically, the patient with PV presents with erythrocytosis, leukocytosis, thrombocytosis, splenomegaly, and positivity for JAK2 V617F . The bone marrow biopsy shows hypercellularity with trilineage hyperplasia. In patients with only some of the characteristics listed earlier, it is important to determine the arterial oxygen saturation using an arterial blood gas and the carboxyhemoglobin level to rule out smokers polycythemia. The P 50 o 2 should be established in patients with other family members with erythrocytosis to exclude congenital disorders with high-oxygen-affinity hemoglobins leading to tissue hypoxia and erythrocytosis. A Pa o 2 greater than 67 mm Hg or an O 2 saturation greater than 95% on an arterial blood gas helps to rule out hypoxic conditions that lead to erythrocytosis. Other essential tests are serum EPO levels, the ability of bone marrow cells to form erythroid colonies in the absence of exogenous EPO, and JAK2 V617F determination. Increase of EPO levels with erythrocytosis indicates a hypoxic cause of secondary erythrocytosis, whereas extremely low levels of EPO (<4 mIU/mL) are virtually diagnostic of PV.
Clinical criteria for diagnosis of PV have been defined by the World Health Organization (WHO) and include molecular testing for JAK2 mutations and histopathologic parameters ( Box 2 ). Individual patients may not meet the diagnostic criteria for PV as defined by WHO, but may clearly have a PV-like MPN.