Neutropenia is a common reason for hematology consultations in the inpatient and outpatient settings and is defined as an absolute neutrophil count less than 1500 cells/μL. Neutropenia varies in severity, with more profound neutropenia being associated with higher rates of infections and infection-related deaths. The causes for neutropenia are diverse and include congenital and acquired conditions (ie, autoimmune, drugs, infection, and malignancy). This article outlines the most common causes of neutropenia and discusses differential diagnoses, treatment modalities, and the mechanisms by which neutropenia occurs.
Neutrophil overview
Neutrophils (also called granulocytes) are produced exclusively in the bone marrow during normal conditions. Approximately 10 12 neutrophils are produced per day in the bone marrow and then stored in the marrow until prompted for release by chemokines, cytokines, microbial products, or other mediators of inflammation. Once released into the bloodstream the average half-life of neutrophils is 6 to 8 hours. Circulating neutrophils, the ones reported in a standard complete blood count (CBC), account for only 2% to 3% of all neutrophils. Clearance occurs in the liver, spleen, or bone marrow and occurs through macrophage phagocytosis of aged or apoptotic neutrophils. The local production of inflammatory cytokines and chemokines leads to neutrophil attachment to the vascular endothelium and the subsequent transmigration of neutrophils into tissue. The migration of neutrophils into tissue is a key component of the innate immune system, as evident by the increased risk of infections seen in the setting of neutropenia.
Neutropenia
Neutropenia is defined as an absolute neutrophil count (ANC) less than 1500 cells/μL; it may be mild (ANC 1000–1500 cells/μL), moderate (500–1000 cells/μL), or severe (<500 cells/μL) ( Table 1 ). In general, infection risk increases with ANC less than 1000 cells/μL; however, the risk for infections varies depending on the cause of neutropenia. For example, patients with neutropenia and acute leukemia seem to have a high risk for overwhelming infection in the setting of neutropenia, particularly in cases with ANC less than 500 cells/μL. Therefore, the context in which neutropenia occurs must be considered because some causes of neutropenia, namely ethnic neutropenia and chronic idiopathic neutropenia (CIN), have few overall infection risks.
| ANC | Risk of Infection | |
|---|---|---|
| Mild neutropenia | ANC <1500 but >1000 | Mild |
| Moderate neutropenia | ANC <1000 but >500 | Moderate |
| Severe neutropenia | ANC <500 but >200 | Severe |
| Agranulocytosis | ANC <200 | Severe |
Neutropenia
Neutropenia is defined as an absolute neutrophil count (ANC) less than 1500 cells/μL; it may be mild (ANC 1000–1500 cells/μL), moderate (500–1000 cells/μL), or severe (<500 cells/μL) ( Table 1 ). In general, infection risk increases with ANC less than 1000 cells/μL; however, the risk for infections varies depending on the cause of neutropenia. For example, patients with neutropenia and acute leukemia seem to have a high risk for overwhelming infection in the setting of neutropenia, particularly in cases with ANC less than 500 cells/μL. Therefore, the context in which neutropenia occurs must be considered because some causes of neutropenia, namely ethnic neutropenia and chronic idiopathic neutropenia (CIN), have few overall infection risks.
| ANC | Risk of Infection | |
|---|---|---|
| Mild neutropenia | ANC <1500 but >1000 | Mild |
| Moderate neutropenia | ANC <1000 but >500 | Moderate |
| Severe neutropenia | ANC <500 but >200 | Severe |
| Agranulocytosis | ANC <200 | Severe |
Diagnostic workup
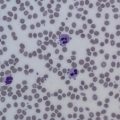
Initial workup consists of a CBC, with a differential count to evaluate the severity of the neutropenia. A full history is also essential to determine race, ethnicity, new medications (including over-the-counter and complementary medications), and potential infectious exposures. Review of systems should focus on fevers, chills, night sweats, weight loss, excess bleeding or bruising, or recurrent infections. A comprehensive physical examination should be performed, with a focus on an examination for signs of infection, hepatosplenomegaly, and lymphadenopathy. After this examination, a detailed review of the peripheral smear should follow, to look for neutrophil abnormalities such as Döhle bodies (infection), immature neutrophil precursors (infection, myelodysplasia, myelopthisis), hypoplastic changes in the neutrophils (myelodysplasia), hyperlobulation (nutritional deficiencies), and white cell inclusions (eg, anaplasmosis ( Fig. 1 ), bartonellosis). Review of red cell morphology on peripheral smear may also offer clues to the cause of neutropenia because dacrocytes (teardrop cells) and nucleated red cells (myelodysplasia, fibrosis, myelopthisis) in addition to red cell inclusions (eg, babesiosis, malaria) may all be seen in disease states associated with neutropenia.
Additional routine blood work should include:
- •
Reticulocyte count
- •
Lactate dehydrogenase
- •
Erythrocyte sedimentation rate
- •
Rheumatoid factor/anticyclic citrullinated protein antibody
- •
Antinuclear antibodies
- •
Thyroid-stimulating hormone
- •
Human immunodeficiency virus (HIV) enzyme-linked immunosorbent assay (ELISA) test with confirmation by Western blot
- •
Vitamin B 12 levels
- •
Folate levels.
In cases in which diarrhea is present, stool samples should be checked for the presence of fecal leukocytes and cultured for Shigella and Salmonella . If tick-borne illnesses are part of the differential diagnosis, then an ELISA for Borrelia burgdorferi , the causative agent of Lyme disease, should be ordered and, if positive, the result should be confirmed by Western blot. If babesiosis is suspected, Babesia immunofluorescent antibody (IFA) and polymerase chain reaction (PCR) tests should be ordered. Anaplasma infection should be evaluated, if needed, by IFA, ELISA, or PCR tests. The granulocyte agglutination test or granulocyte immunofluorescence test for antigranulocyte antibodies to evaluate potential autoimmune neutropenic syndromes may be considered. Bone marrow aspirate and biopsy should be reserved for those cases in which there is a high suspicion for malignancy, either hematologic or metastatic solid tumor.
Causes
The most common causes of neutropenia are shown in Boxes 1–3 .
- 1.
Ethnic variations
- 2.
Immune-related
- a.
Primary immune neutropenia
- b.
Secondary immune neutropenia
- i.
Felty syndrome (FS)
- ii.
Systemic lupus erythematosus (SLE)
- iii.
Rheumatoid arthritis (RA)
- i.
- a.
- 3.
Infectious
- a.
Sepsis
- b.
Parasitic
- c.
Viral
- d.
Bacterial
- a.
- 4.
Malignancy
- a.
Acute leukemia
- b.
Myelodysplastic syndrome (MDS)
- c.
Myelophthisis
- d.
Large granulocytic lymphocytic leukemia
- a.
- 5.
Medication
- a.
Antibiotics
- b.
Cardiac
- c.
Anticonvulsants
- d.
Psychiatric
- e.
Antiinflammatory
- f.
Hypoglycemics
- g.
Antineoplastic agents
- a.
- 6.
Mechanical
- a.
Splenomegaly
- a.
- 7.
Nutritional deficiencies
- a.
Vitamin B 12 (cobalamin)
- b.
Folic acid
- c.
Copper
- a.
- 8.
Other
- a.
Hypothyroidism/hyperthyroidism
- a.
- 1.
Severe congenital neutropenia (SCN)
- 2.
Cyclic neutropenia
- 3.
WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome
- 4.
Shwachman-Diamond syndrome
- 5.
Barth syndrome
- 6.
Pearson syndrome
- 7.
Glycogen storage disease type 1B
- 8.
Cartilage-hair hypoplasia
- 9.
Dyskeratosis congenita
- 1.
Sepsis
- 2.
Parasitic
- a.
Malaria
- b.
Leishmaniasis (kala-azar)
- c.
Babesiosis
- a.
- 3.
Viral
- a.
HIV
- b.
Human herpesviruses
- i.
Herpes simplex virus 1 and 2
- ii.
Varicella zoster virus
- iii.
Epstein-Barr virus
- iv.
Cytomegalovirus (CMV)
- v.
Roseolovirus (sixth disease)
- i.
- c.
Parvovirus B19 (fifth disease)
- d.
Hepatitis A, B, and C virus
- e.
Measles
- f.
Rubella
- a.
- 4.
Bacterial
- a.
Shigella
- b.
Salmonella (typhoid fever)
- c.
Zoonotic diseases (tularemia, brucellosis)
- d.
Anaplasmosis
- e.
Lyme disease
- a.
- 5.
Mycobacterial
- a.
Mycobacterium tuberculosis
- b.
M avium
- a.
Ethnic variation
Ethnic neutropenia has been described in persons of African, African American, Yemenite Jewish, West Indian, and Arab Jordanian ancestry. In the United States, the prevalence of neutropenia (ANC <1500 cells/μL) amongst African Americans is 4.5%, whereas it has been reported at 0.79% and 0.38% in whites and Mexican-Americans, respectively. The pathophysiology in ethnic neutropenia seems to be a decrease in the number of neutrophil precursors within the bone marrow. However, there is no decrease in the functionality of the neutrophils. Ethnic neutropenia is a benign process that carries no increased risks for infections but should be considered early in the initial diagnostic workup to prevent unnecessary testing. Recently, the role of ethnic neutropenia has been addressed in patients receiving chemotherapy, because most clinical trials withhold chemotherapy in the setting of an ANC less than 1500 cells/μL. Hsieh and colleagues make the case that chemotherapy can be administered with granulocyte colony-stimulating factor (G-CSF) support, if appropriate, in patients with ethnic neutropenia at ANC of 500 to 1500 cells/μL.
Congenital neutropenia
The hematologist who treats adults should also be familiar with congenital forms of neutropenia (see Box 2 ). Cases can be mild and therefore not diagnosed until patients reach their adult years and have more regular blood work performed. Moreover, patients with severe forms of congenital neutropenia are living longer and require transitions from pediatric hematologists to their adult counterparts. The 2 most common forms of congenital neutropenia, cyclic neutropenia and severe congential neutropenia (SCN, or Kostmann syndrome), are reviewed here with other causes of congenital neutropenia.
Cyclic Neutropenia
Typically, in cyclic neutropenia a family history is important because the condition occurs through autosomal-dominant inheritance, although sporadic cases have been reported. The neutrophil count cycles over an approximately 21-day period. Patients commonly develop oral ulcerations, fevers, cervical lymphadenopathy, and skin infections during neutrophil nadir periods, but severe infections are usually not seen. The disorder is secondary to a mutation in the neutrophil elastase gene that leads to increased apoptosis in neutrophil precursors. Treatment, if required, is centered on G-CSF with good overall results. The level of neutropenia sometimes improves with age.
Severe Congenital Neutropenia (Kostmann Syndrome)
Initially described by the Swedish physician Rolf Kostmann in 1956 in a cohort of children who presented with agranulocytosis and severe infections. SCN was, at first, believed to occur through autosomal-recessive inheritance. However, autosomal-dominant inheritance and sporadic cases of SCN have also been reported. Like cyclic neutropenia, mutations in the neutrophil elastase gene have been found in patients with SCN, although bone marrow biopsy shows maturation arrest at the promyelocyte stage. However, the role of these mutations remains unclear because they were found in a subgroup of patients with SCN but were also found in phenotypically normal family members. HAX1 gene mutations have been associated with autosomal-recessive forms of SCN, whereas ELA2 mutations are commonly found in autosomal-dominant and sporadic cases of the disease. More recently, a mutation in the G6PC3 gene has also been shown to result in SCN. More than 90% of the patients respond to G-CSF administration. Long-term complications include a risk for transformation to myelodysplasia or leukemia in 22% of patients. This transformation has been unmasked now that patients live longer because of G-CSF therapy. Vasculitis, splenomegaly, and osteoporosis frequently occur in this patient population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree