Whole-Breast Radiation Following Breast-Conserving Therapy for Noninvasive and Invasive Cancers: Introduction
Breast-conserving therapy (BCT), consisting of surgical removal of the primary tumor followed by radiation therapy to the intact breast, is now considered a standard treatment approach for early-stage breast cancer. Increasing numbers of patients are becoming eligible for breast conservation after the diagnosis of breast cancer, due to improvements in early detection and patient awareness. In addition, patients presenting with larger tumors that previously would have required mastectomy may now be eligible for BCT using neoadjuvant chemotherapy to downsize the tumor, followed by local excision and radiation. Data from multiple randomized prospective studies have demonstrated equivalent long-term survival outcomes for BCT compared to mastectomy and the benefit of adding adjuvant radiation therapy after conservative surgery. Furthermore, there are numerous retrospective single-institutional series that support the use of conservative surgery and radiation therapy in early-stage breast cancer. This chapter will discuss the indications and validity of postoperative, whole-breast radiation therapy for noninvasive and invasive cancer, and will address special considerations and future directions for radiation therapy as a component of breast conservation therapy for early-stage breast cancer.
Lobular carcinoma in situ (LCIS) is generally considered a risk indicator for the development of invasive carcinoma, and not a malignancy in and of itself. Although the overall management of pure LCIS remains somewhat controversial, patients are often treated with conservative surgery alone or sometimes mastectomy. In addition, there are substantial data to support the use of tamoxifen for LCIS, to reduce the risk of future invasive breast cancer.1 In patients who undergo surgical excision with pure LCIS histology, the literature on the use of radiotherapy postoperatively is very limited.2,3 After a recent workshop on LCIS conducted by EUSOMA members in London, the group concluded that “there is little data to recommend radiation therapy in the clinical management of LCIS.”4 Therefore, based on insufficient data to support its usage, radiation therapy after local excision is not typically utilized in the management of pure LCIS.
For patients who have LCIS as a component of their invasive breast cancer, the decision for BCT should be based on the evaluation of the invasive component alone. If LCIS is present at the surgical margin of an invasive carcinoma, no further re-excision is required so long as the invasive component is completely excised. Despite some conflicting data from single-institutional series,5-7 it is generally accepted that local control does not appear to be compromised with LCIS as a component of invasive cancer. Therefore, BCT is a reasonable treatment option for patients with invasive cancers with associated LCIS, and further excision for margins involved with LCIS alone is not warranted.
Although ductal carcinoma in situ (DCIS) is a noninvasive breast malignancy with an overall good to excellent prognosis, the biological diversity correlates with the variable malignant potential and therefore the risk of local recurrence can be relatively high with surgery alone. In addition, the natural history of recurrent disease can be significantly different from the primary DCIS, with a risk of approximately 50% of the recurrences being invasive disease, with a higher potential for nodal and distant metastasis. For these reasons, preventing recurrence for DCIS is of paramount importance. The local treatment approaches for DCIS include the following: (1) simple mastectomy, (2) breast-conserving surgery and radiation therapy, and (3) breast-conserving surgery alone. The rationale for treatment with breast-conserving surgery and radiation over conservative surgery alone (without radiation) has been substantiated by several large, randomized trails. The diversity in histologic features and differences in malignant potential of DCIS suggests that a proportion of patients may be candidates for surgical excision alone with omission of radiation, yet at this juncture, no prospective studies have been able to identify subgroups of patients who may be potential candidates for observation after surgery. The current standard of care, based on the results of these large randomized, prospective studies, indicates that radiation therapy after breast-conserving surgery significantly reduces the local relapse rates in all subsets of patients with DCIS, decreasing the risk of both invasive and noninvasive recurrences.
The prospective study with the longest follow-up comes from the National Surgical Breast and Bowel Project (NSABP). The NSABP B-17 addressed the issue of radiation for DCIS by randomizing 818 patients to lumpectomy alone versus lumpectomy plus radiation therapy.8 Patients were randomized postoperatively to a standard dose of 50 Gy in 25 fractions over 5 week to the whole breast versus observation. All patients were required to have negative margins. After a median follow-up of 10.75 years, the patients randomized to lumpectomy alone had a relapse rate of 32% versus 16% in patients treated with adjuvant radiation. Approximately 50% of the recurrences in both cohorts were invasive cancers; thus, radiation significantly reduced the risk of invasive and in situ recurrences. There were no differences observed in overall survival with the addition of radiation.
The European Organization for Research and Treatment of Cancer (EORTC) also conducted a landmark study addressing the role of radiation therapy for DCIS less than 5 cm in size resected with negative surgical margins,9 similarly randomizing patients to observation versus postoperative radiation therapy. Their findings were consistent with the NSABP B-17 trial, resulting in a statistically significant benefit in local control for DCIS treated with radiation therapy at 10.5 years in all subgroups of patients.
A similarly conducted randomized study of postoperative radiation for DCIS from Sweden (the SweDCIS Trial) found a cumulative incidence of recurrence of 0.07 (95% CI 0.050-0.10) in the radiation arm compared with 0.22 (95% CI 0.18-0.26) in the observation arm at a median follow-up of 5.2 years.10 This corresponded to an overall hazard ratio of 0.33 (95% CI 0.24-0.47, p < 0.0001). Although their findings were consistent with the studies mentioned earlier, this study differed from those trials in that the protocol did not require microscopically negative margins prior to radiation; 10% of the patients had positive surgical margins in this study.
The fourth landmark study was conducted in the United Kingdom, Australia, and New Zealand (UK/ANZ DCIS trial), and randomized DCIS patients to 1 of 4 arms after surgery: postoperative radiation, postoperative tamoxifen, a combination of radiation and tamoxifen, or observation.11 The findings of this study also favored the use of radiation therapy for DCIS, with local recurrences of 8% and 6% for the radiation arms, and 22% and 18% for the nonradiation arms, at a median follow-up of 4.4 years. Again, adjuvant radiation was associated with a statistically significant reduction of in-breast recurrences (HR = 0.38, p < 0.0001), with a significant reduction in both invasive and noninvasive cancers. There was no apparent benefit to tamoxifen for DCIS in this study. The findings of these 4 randomized studies are detailed in Table 94-1.
| Trial | Trail Design | No. Patients | Med F/U (y) | LC Outcomes |
|---|---|---|---|---|
| NSABP B-17 | RT vs Observ. | 818 | 12 | 16% vs 32%* |
| EORTC | RT vs Observ. | 1010 | 10.5 | 15% vs 26%a |
| SweDISH | RT vs Observ. | 1067 | 5.2 | 7% vs 22%a |
| UK/ANZ | RT vs RT + Tam vs. Tam vs Observ. | 1701 | 4.4 | 8% vs 6% vs. 18% vs 22%* |
More recently, a meta-analysis of the 4 randomized trials was conducted, pooling the 3665 patients together to determine the cumulative benefit of adjuvant radiation therapy for DCIS.12 Their findings showed a significant reduction of invasive and in-situ ipsilateral breast cancer, with an odds ratio (OR) of 0.40 (95% CI 0.33-0.60, p < 0.00001) and 0.40 (95% CI 0.31-0.53, p < 0.00001), respectively. As expected, distant metastases and death rates between the 2 arms did not differ. Interestingly, there were more contralateral breast cancers after adjuvant RT in comparison to the observation arm, with a 1.53-fold higher likelihood of contralateral breast cancer (95% CI 1.05-2.24, p = 0.03). Subset analysis did not isolate any DCIS patient groups in whom radiation could be omitted.
All of randomized trails discussed in this section treated patients to 50 Gy in 2-Gy fractions without a boost. Based on these data, radiation therapy after local excision for DCIS has been shown to decrease ipsilateral breast tumor recurrences by approximately 60%; this decrease includes both in situ and invasive relapse. No difference in overall survival has been demonstrated with the addition of radiation therapy. At this juncture, this benefit in local control appears to apply broadly to all subsets of patients with DCIS.
There is a clinically relevant need to identify subsets of DCIS patients in whom postoperative radiation may be omitted; thus, the issue of subjecting all DCIS patients to radiation remains an area of controversy, particularly because no survival benefit has been noted. Some institutions continue to stratify patients according the Van Nuys Prognostic Index,13 a system in which patients are given a score based on margin status, histologic subtype, tumor size, and patient age (Table 94-2). Based on the retrospective analysis of the a cohort of DCIS patients from 2 institutions, the authors of this scoring system recommend breast-conserving surgery alone (postoperative observation) for scores of 4 to 6, surgery plus radiation for scores of 7 to 9 (radiation decreased local recurrence by a statistically significant difference of 15%-12%), and mastectomy for scores of 10 to 12 (because the recurrence rate with breast-conserving surgery and radiation were unacceptably high at 50% at 5 years in this subgroup). From a clinical standpoint, though the basis for utilizing a “scoring system” for DCIS makes sense, it is important to note that none of the randomized data, either alone or pooled, have been able to isolate pathologic or patient criteria for omission of radiation for DCIS for DCIS. Furthermore, a recent, single-arm prospective study of observation after breast-conserving surgery for DCIS (wide excision alone with larger than 1-cm margins for small, low-grade tumors) was closed early to accrual because of the high number of local recurrences with observation alone.14
The topic of excision alone for DCIS will need to continue to be studied in a prospective fashion. Radiation Therapy Oncology Group (RTOG) trial 98-04 was designed to address this question by randomizing low-risk DCIS patients to lumpectomy alone versus lumpectomy plus radiation. Unfortunately, the trial closed due to poor patient accrual. The European Cooperative Oncology Group (ECOG) E-5194 is a prospective study of approximately 1000 DCIS patients (< 2.5 cm low to intermediate grade, or < 1 cm high grade) treated with excision and negative margins larger than 3 mm, with the end points of local recurrence at 5 and 10 years. The result of this study should provide useful information on the efficiency of lumpectomy alone for low-risk DCIS. Currently, it remains unclear how to identify patients with DCIS who can be treated with excision alone. Although future studies may identify patients with DCIS who can be treated with observation after breast-conserving surgery the existing prospective data suggest a significant benefit for radiation therapy for low-, intermediate-, and high-risk groups, based on our current classifications. Future molecular profiling studies may help to stratify patients into risk categories to identify subsets of patients in whom radiation may be omitted.
The surgical management of early-stage, invasive breast cancer has significantly evolved over the last several decades. Historically, local control of the primary tumor was achieved by removal of the entire breast. We now know that, in appropriately selected patients, removing the primary tumor with a rim of surrounding normal tissue (breast-conserving surgery), followed by radiation therapy to the whole breast, provides equivalent long-term outcomes to mastectomy and offers the patient the opportunity to preserve the breast. Long-term data from large, randomized prospective studies have validated the use of breast-conservation therapy as a standard treatment option for early-stage breast cancer. These studies have demonstrated that less extensive surgery does not compromise breast cancer outcomes when the tumor is completely removed with negative margins and followed by postoperative radiation therapy to the intact breast. Over the last decade, BCT has gained acceptance as a comparable alternative to mastectomy for early-stage breast cancer; in 1991, the National Cancer Institute (NCI) issued a consensus statement stating that “BCT is an appropriate method of primary therapy for the majority of women with stage I and II breast cancer and is preferable because it provides survival equivalent to total mastectomy and axillary dissection.”15 As the efficacy of BCT becomes better understood by both physicians and patients, the rates of BCT in the United States continue to rise.
The goal of BCT should be to achieve local recurrence of upto 1% per year, based on the recommendations of the Consensus Conference of Breast Conservation.16 While defining the appropriate selection of patients for BCT remains controversial, established criteria are based on the ability to (1) achieve an acceptable cosmetic result (ie, tumor size with respect to breast size), (2) obtain negative margins at surgery, and (3) safely deliver radiation therapy and minimize potential for side effects. Meticulous patient selection, surgical techniques, and radiation delivery are therefore required to optimize local control.
There are numerous prospective studies that have been conducted worldwide addressing the role of BCT in early-stage breast cancer. There are 6 modern prospective randomized trials17-22 comparing mastectomy to conservative surgery plus radiation therapy (Table 94-3). All of these trials have demonstrated that there is no significant difference in distant metastasis, cause-specific survival, or overall survival between mastectomy and BCT. In addition, none of these studies found a difference in contralateral breast cancers or second malignancies between the 2 cohorts.
| Trial | Follow-up (y) | No. Patients | OS |
|---|---|---|---|
| NSABP-B0617 | 20 | 1219 | NS |
| Milan18 | 20 | 701 | NS |
| Institute Gustave-Roussy19 | 15 | 179 | NS |
| National Cancer Institute20 | 10 | 237 | NS |
| Danish Breast Cancer Group21 | 6 | 905 | NS |
| EORTC22 | 10 | 868 | NS |
Given that the efficacy of BCT compared to mastectomy has been proven, it follows that the value of adding radiation to lumpectomy alone needs establishment. Multiple randomized prospective studies17,23-29 have assessed the role of radiation therapy after breast-conserving surgery (Table 94-4). These studies randomized patients to surgical excision alone versus excision followed by radiation (± adjuvant systemic therapy or tamoxifen). Overall, each of these studies has demonstrated a statistically significant improvement in local control when adding adjuvant radiation therapy to breast-conserving surgery, with an approximately two-thirds reduction in local recurrence. None of the studies have shown that the addition of radiation therapy has resulted in an improvement in survival. Based on the current available data from these randomized prospective trials, radiation therapy after local excision is the standard of care for the majority of patients who choose to conserve their breast. The benefit in local control with the addition of radiation therapy after conservative surgery from the NSABP B-06 20-year follow-up is shown in Figure 94-1. The guidelines by the American College of Radiology (ARS) for BCT in stage I and II patients currently state that “whole breast radiation with or without boost is the standard of care after lumpectomy.”30
| Trial | No. Patients | Local CS (%) | Relapse CS + RT (%) | F/U (y) |
|---|---|---|---|---|
| NSABP B-0617 | 1265 | 41 | 12 | 10 |
| NSABP B-2123 | 1006 | 17 | 3 | 8 |
| Ontario COG27 | 837 | 35 | 11 | 7.6 |
| Milan III28 | 579 | 24 | 6 | 10 |
| Scottish24 | 556 | 5 | ||
| ER+ | 25 | 3 | ||
| ER− | 44 | 14 | ||
| British29 | 399 | 35 | 13 | 5 |
| Swedish24 | 585 | 24 | 6 | 6 |
| Uppsala25 | 381 | 18 | 2 | 5 |
| West Midlands, UK26 | 707 | 18 | 8 | 4.2 |
Figure 94-1
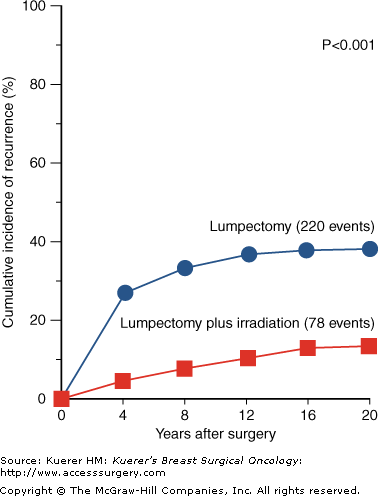
Twenty-year follow-up from NSABP B-06 trial showing cumulative incidence of a first recurrence of cancer the ipsilateral breast in patients treated with lumpectomy alone versus lumpectomy plus radiation therapy. (Reproduced with permission from Fisher B, Anderson S, Margolese RG, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241.)
In 2004, a pivotal meta-analysis was published of 15 randomized studies evaluated lumpectomy plus radiation versus lumpectomy alone.31 Although none of the individual studies assessing the role of radiation after lumpectomy suggested a benefit in survival, this analysis found a small but statistically significant benefit in overall survival with the addition of radiation. In this pooled analysis, 9422 patients were evaluated and as expected, the relative risk of developing an ipsilateral breast tumor recurrence without radiation compared to with radiation was 3.00 (95% CI 2.65-3.40). Furthermore, the relative risk of mortality was 1.086 (95% CI 1.003-1.175), corresponding to an estimated 8.6% (95% CI 0.3-17.5) relative excess mortality if radiotherapy was omitted.
Another landmark meta-analysis performed by the Early Breast Cancer Trialist’s Cooperative Group combined data from 42,080 patients from prospective randomized trials addressing the role of radiation for breast cancer.32 For the subset of patients who had undergone BCT (7311 patients from 10 trials), postoperative radiation therapy was associated with a statistically significant absolute reduction in local recurrence of 19% at 5 years (decrease in local relapse from 26% to 7%). When pooling the data collectively, this translated into an absolute reduction in breast-cancer-specific mortality of 5.4% at 15 years (Fig. 94-2). Radiation produced similar proportional reduction in local relapse in all women, regardless of age or pathologic characteristics. It was notable that these data demonstrated a 1.8% increase in second malignancies (contralateral breast and lung cancers) and a 1.3% increase in deaths from causes other than breast cancer (particularly heart disease and lung cancer), drawing attention to the toxicities of radiation and the necessity to carefully plan and deliver radiation to minimize the dose to surrounding normal tissue. Despite these additional risks from radiotherapy, the absolute reduction in 15-year overall mortality was similar to the 15-year breast-cancer-specific mortality. The authors concluded that for every 4 local recurrences avoided by optimizing local control, 1 breast cancer death would be avoided. This very important study highlights the relationship between local recurrence and survival, suggesting that despite the increased death from other causes for patients who received radiation, there is an association between improved local control with the addition of radiation therapy and improvement in overall mortality.
Figure 94-2
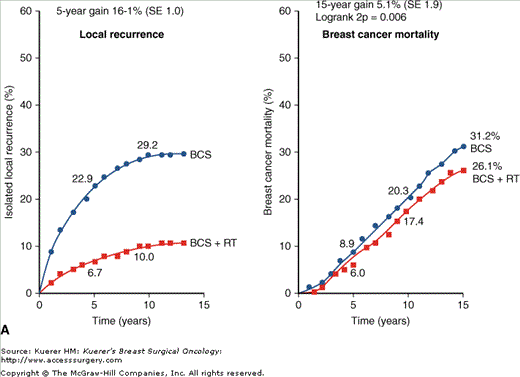
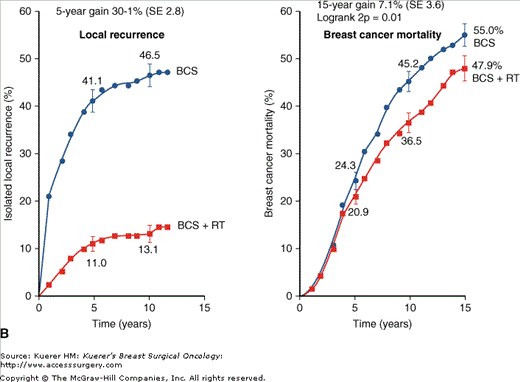
A. Local recurrence and breast cancer mortality improvements with the addition of radiation therapy to breast-conserving surgery in node-negative patients. B. Local recurrence and breast cancer mortality improvements with the addition of radiation therapy to breast-conserving surgery in node-positive patients. (Reproduced with permission from Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-2106.)
Several well-designed trials have addressed the role of omitting radiation after breast-conserving surgery for subsets of patients with good prognostic features in whom the absolute benefit of radiation may not be clinically apparent. A trial from Canada attempted to omit radiation in patients over the age of 50 with node-negative, estrogen receptor (ER)-positive tumors less than 5 cm.33 Patients were randomized to tamoxifen alone versus radiation plus tamoxifen. The rate of local relapse at 5 years was 7.7% in the tamoxifen group and 0.6% in the tamoxifen plus irradiation group (p < 0.001), with a corresponding difference in 5-year disease-free survival rates of 84% and 91% (p = 0.004). In addition, the axillary relapse rate was significantly higher in patients not receiving radiation. Even with subset analysis of the smaller tumors (< 2 cm), the relapse rates were unacceptably high with the omission of radiation, with 5-year local relapse of 0.4% in the tamoxifen plus radiotherapy arm and 5.9% in the tamoxifen alone arm (p < 0.001). Based on these data, there is no justification for omission of radiation therapy in this subset of patients more than 50 years of age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree