Traditionally, neoadjuvant chemotherapy has been used to downstage locally advanced and unresectable primary breast tumors to make them operable.1,2 Endocrine therapy has emerged as an attractive alternative in the neoadjuvant setting for postmenopausal women with estrogen receptor–positive (ER+) breast cancer, with comparatively less toxicity. Direct comparison between neoadjuvant chemotherapy and endocrine therapy has not been addressed in large randomized studies as the selection of patients for either treatment has to date been based upon menopausal status and ER status of the tumor. One Russian study randomized 121 postmenopausal women with T2-4 ER+ and/or progesterone–receptor-positive (PR+) breast cancer to receive either neoadjuvant doxorubicin and paclitaxel chemotherapy (n = 62) or neoadjuvant endocrine treatment with anastrozole (n = 30) or exemestane (n = 29) for 3 months prior to surgery.3 Clinical and mammographic objective response rates (ORR) were similar for endocrine therapy and chemotherapy, and there was a trend for greater rates of breast-conserving surgery with endocrine therapy with no significant differences in local recurrence rates at 34 months. Grade 3/4 alopecia, neutropenia, cardiotoxicity, and neuropathy were experienced by significant numbers of women in the chemotherapy group. Neoadjuvant endocrine therapy was better tolerated, the most common adverse events being hot flushes, fatigue, vaginal bleeding, and arthralgia. These data support the view that endocrine therapy is a safe alternative to chemotherapy, with similar response rates but less toxicity. In ER+ breast cancer there is also some evidence to suggest that neoadjuvant chemotherapy is less effective than in ER– breast cancer. A recent retrospective study of 1731 patients who had primary chemotherapy showed a significantly lower rate of pathologic complete response to chemotherapy in patients with hormone receptor–positive tumors compared with those with hormone receptor–negative tumors (8% vs 24%; p < .0001).4 These data are consistent with the observation that patients with hormone receptor–positive disease are less chemosensitive and respond better to endocrine therapy. There is also evidence to suggest that the histological response to chemotherapy and endocrine therapy differ, with higher rates of central scarring in tumors treated with neoadjuvant letrozole (58.5% vs 2%, p = .035).5 Central scarring correlates with clinical response and explains why, following endocrine therapy, excising the residual mass results in complete excision more often than after neoadjuvant chemotherapy where scattered widespread foci of disease are significantly more common.5 In this study, complete pathological response rate, as expected, was seen significantly more frequently after neoadjuvant chemotherapy, although this was not seen in the randomized study by Semiglazov.3
With careful selection there are benefits of neoadjuvant therapy compared with primary surgery. The most significant benefits are in postmenopausal patients with large operable or locally advanced inoperable ER+ tumors, which can be downstaged to become operable or to be suitable for less extensive surgery, such as breast conservation in those originally thought to require mastectomy. It is widely accepted that breast conservation followed by radiotherapy provides improved quality of life, better cosmetic outcomes, and comparable disease control compared with mastectomy, although this has yet to be proven in any long-term study following neoadjuvant therapy. The majority of patients who are spared mastectomy are elderly but studies have shown that older women are no more likely to choose mastectomy than younger women if given a choice.
In early studies with tamoxifen patients were not selected on the basis of ER or PR status. It has since been shown that ER positivity is an important predictor for response to tamoxifen and aromatase inhibitors.6 Selection of patients for neoadjuvant endocrine therapy in patients with ER+ tumors needs to be based upon the likely benefit that can be obtained from such treatment. Patients who are likely to benefit are those with locally advanced breast cancer who may, after response, become operable; those with large operable tumors that have the potential for conversion from mastectomy to breast-conserving surgery if the tumor volume can be reduced; and those with a large cancer suitable for breast-conserving surgery whose cosmetic outcome may be improved by allowing a less extensive local excision with tumor shrinkage. Patients who are unlikely to benefit are (1) those who have operable disease that is multifocal that usually requires mastectomy regardless of response with treatment and (2) patients with certain tumor types that have been shown to respond slowly or indiscernibly to treatment such as invasive lobular carcinomas (ILCs) and mucinous carcinomas. Response in such patients can be difficult to assess. There is a need in patients with ILC to assess extent of disease accurately prior to treatment, and in mucinous cancers to consider longer durations of treatment, which are often necessary to see shrinkage in such cancers.
Tamoxifen was the first widely used neoadjuvant endocrine therapy, but there have been a number of studies published recently investigating the role of aromatase inhibitors in this setting. The results and outcomes of these studies are discussed below and provide evidence upon which treatment decisions can be based.
Most of the studies using tamoxifen as a primary treatment have compared surgery with or without tamoxifen to the use of tamoxifen alone, and thus were not designed to assess tamoxifen in the true neoadjuvant setting. These studies do, however, show the effect of tamoxifen as a primary treatment. There have been 3 large randomized trials of tamoxifen alone versus surgery alone. While 1 trial found no difference between the 2 treatments at 6 years,7 a Nottingham study found a significant increase in local progression in the tamoxifen-alone group at a median follow-up of 145 months.8 This was confirmed in a multicenter European study that also reported a shorter time to progression and shorter disease-free survival, as well as poorer locoregional control in the tamoxifen-alone group at a median 120 months follow-up.9
Three randomized trials have compared tamoxifen alone with surgery followed by tamoxifen. In an Italian study, 474 patients were randomized to surgery followed by 5 years tamoxifen or to 5 years tamoxifen alone.10 No difference was found in overall survival or breast-cancer-specific survival at 80 months, but there was a significantly higher local progression rate in the tamoxifen-only arm (106 vs 27; p = .0001). In the 2 studies that have reported follow-up to 12 years there was a significant increase in the rate of local recurrence in the tamoxifen-alone group, and a significantly higher overall mortality and mortality from breast cancer in the tamoxifen-only groups, although in 1 of these 2 studies the increase in mortality was observed only after 3 years.11,12 In none of the randomized trials were patients selected on the basis of ER status, and this will have influenced outcome as ER-negative patients will have gained no benefit from tamoxifen. One conclusion from these studies was that using long-term tamoxifen treatment without surgery should be reserved for patients in whom life expectancy is short (2-3 years maximum).11
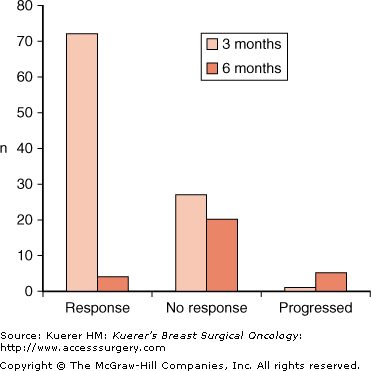
These studies did not clarify how long tamoxifen is effective when given as the sole therapy to women with hormone-sensitive cancer. One study did report long-term follow-up data for 113 patients over the age of 70 who had been treated with tamoxifen as the sole therapy.13 The median time to failure of local control was 2.5 to 3 years, with more than half of the responders relapsing by 5 years. In Edinburgh, a series of 100 women more than 70 years of age, with ER+ tumors, were treated to investigate the time course of response with neoadjuvant tamoxifen.14 Modified World Health Organization (WHO) criteria were used to evaluate tumor response in the neoadjuvant setting as follows:
- Partial response (PR): reduction in tumor size 50% or more from pretreatment size
- Minor response (MR): reduction in tumor size 25% or more and less than 50% from pretreatment size
- No change (NC): less than 25% decrease or less than 25% increase in tumor size from pretreatment size
- Complete response (CR): no measurable tumor
- Progressive disease (PD): 25% or more increase in tumor size from pretreatment size
After 3 months of tamoxifen, 72 patients had responded (CR, PR, or MR) and 1 patient had progressive disease (PD). The remaining 27 patients continued on tamoxifen for a further 3 months, during which time 4 responded and 5 progressed (Fig. 84-1). The observation drawn from these data is that it is unlikely that patients who do not have a response by 3 months will have a response with prolonged further treatment, and so if patients do not respond to hormonal therapy within this time period alternative treatments need to be considered.
The first studies using neoadjuvant aromatase inhibitors were performed in Edinburgh and produced promising results,15-17 and have since led to large randomized studies comparing tamoxifen with aromatase inhibitors, the results of which are discussed below.
Tamoxifen has been compared with letrozole in the neoadjuvant setting in a study referred to as the PO24 study, in which 337 postmenopausal women with large ER+ or PR+ breast cancers that required mastectomy or were locally advanced and inoperable were randomized to receive letrozole or tamoxifen for 4 months as primary treatment.18 Objective response rates by palpation, mammography, and ultrasound were assessed, showing a significantly higher clinical response rate in letrozole-treated patients (55% vs 36%; p < .001), and significant results in favor of letrozole on ultrasound and mammograms. There was a significantly higher rate of breast-conserving surgery in the letrozole group than in the tamoxifen group, even in those patients with locally advanced cancer (Fig. 84-2). When these results were analyzed with respect to ER Allred scores, it was evident that not only were response rates to letrozole higher in the high ER score categories (6-8), but they were also higher in patients who had lower ER scores (3-5); among these patients responses were achieved only with letrozole.6,19 This has significant implications for the treatment of patients with tumors with low ER scores who may still benefit from neoadjuvant letrozole treatment if required, whereas tamoxifen may not be as effective in tumors with low levels of ER expression.
Figure 84-2
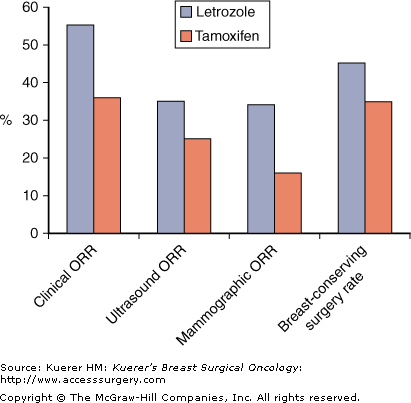
Outcomes from PO24 trial of neoadjuvant letrozole (L) versus tamoxifen (T) for 4 months.18 (L versus T clinical ORR p ≤ .001; ultrasound ORR p = .042; mammographic ORR p < .001; breast-conserving surgery rate p = .022.)
There have been 2 large randomized studies comparing anastrozole with tamoxifen in postmenopausal women with hormone receptor–positive breast cancer.
In the IMPACT (IMmediate Preoperative Arimidex, Tamoxifen or Combined with Tamoxifen), 330 patients from the UK and Germany were randomized to receive anastrozole or tamoxifen or both in combination for 3 months prior to surgery.20 The primary end point was objective clinical response measured by calipers, with ultrasound measurement as a secondary end point. This study found that there was no significant difference in objective response between the 3 treatments as measured by calipers and ultrasound. Among patients with HER-2-positive tumors, anastrozole had a numerically higher clinical response rate than tamoxifen (p = .18). At 3 months, among those patients initially assessed as requiring mastectomy, significantly more were deemed suitable for breast-conserving surgery in the anastrozole arm than tamoxifen arm (“feasible surgery,” 46% vs 22%; p = .03). Not all patients accepted this recommendation, and there was no significant difference in numbers actually undergoing breast conservation (“actual surgery”) between anastrozole and tamoxifen groups at 3 months (44% vs 31%; p = .23).
In the PROACT (PReOperative Arimidex Compared with Tamoxifen) trial the entry criteria were similar to the IMPACT trial, but also included patients whose tumors were inoperable and those on concurrent chemotherapy.21 Patients were randomized to receive either anastrozole (n = 202) or tamoxifen (n = 201), both with placebo, for 3 months. The primary end point was objective tumor response measured by ultrasound scan. Caliper measurement was included as a secondary end point, alongside surgical assessment at baseline and 3 months. No significant difference in ORR was seen between treatment arms in all patients, although a trend was noted in favor of anastrozole in those patients who had hormonal therapy alone. There was, however, a significantly higher ORR in favor of anastrozole in those patients initially assessed as requiring mastectomy (36.6% vs 24.2% on ultrasound, p = .03; and 48.6% vs 35.8% with calipers, p = .04). This also translated into a significant improvement for those initially requiring mastectomy with 43.0% of those treated by anastrozole being treatable by breast-conserving surgery versus 30.8% with tamoxifen (p = .04), and greater numbers of those with initially inoperable tumors having a surgical procedure performed with anastrozole (p = NS).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree