A central tenet of neoadjuvant clinical trials is that tumor response, as a surrogate end point, should be strongly correlated with long-term patient survival.1,2 Otherwise, the value of neoadjuvant treatment would be to convert a tumor to operability or to increase the probability of conservative surgery. However, a close association between pathologic response to treatment and subsequent survival establishes neoadjuvant treatment as a method for clinical trials including operable disease to evaluate promising treatments more purely in terms of direct tumoricidal activity and without confounding variables of natural history and subsequent treatments. Indeed, pathologic complete response (pCR) has been adopted as the primary end point for neoadjuvant trials because it has consistently been associated with long-term survival in neoadjuvant trials using different chemotherapy regimens of variable treatment duration.3-12 If pCR is achieved using current therapies, one can anticipate more than 90% probability of disease-free survival within the first decade of follow-up.
Communication between surgeon, radiologist, and pathologist is very important for accurate definition of pathologic response of a patient’s breast cancer. The macroscopic appearance of residual tumor can be deceptive in a resected breast specimen, so pCR could be falsely ascribed if the residual tumor bed was not correctly identified and sampled, such that a different area of breast tissue was submitted for histopathologic study. Effective use of the pathology requisition form (clinical history section), electronic medical record, placement of metallic indicators in the tumor bed at the beginning of treatment, and conservative surgical resection (lumpectomy) contribute to more accurate gross and microscopic pathologic assessment of response in the primary tumor bed. We find that specimen radiography with radiologic-pathologic correlation is particularly helpful to identify the tumor bed and to map its extent and margin status, particularly if metallic indicators have been placed in the tumor bed.
Although it is generally held that a definition of pCR should include patients without residual invasive carcinoma in the breast (pT0), the presence of nodal metastasis, minimal residual cellularity, and residual in situ carcinoma are not consistently defined as pCR or residual disease (RD).12-15 When there is no residual invasive cancer in the breast, the number of involved axillary lymph nodes is inversely related to survival.11 Conversely, patients who convert to node-negative status after treatment have improved survival, even if there is RD in the breast.16 This supports the concept that pathologic response in the regional lymph nodes is as important as response in the primary tumor bed. Consequently, the combination of tumor size and nodal status after neoadjuvant treatment is prognostic.17 No evidence indicates that residual in situ carcinoma alone increases risk of future distant relapse.12,18,19 However, residual ductal carcinoma in situ (DCIS) is relevant for local control, and so resection specimens are carefully evaluated for residual DCIS and margin status just like any breast cancer specimen. Therefore, the most appropriate definition of pCR with respect to prognosis is lack of residual invasive cancer and node-negative status after neoadjuvant therapy.
Patients who achieve pCR from neoadjuvant (preoperative) systemic therapy have excellent 5-year overall survival that is independent of treatment regimen or tumor phenotype.2,7,9,11,12,20-22 However, it remains difficult to calculate improvements in overall survival for a particular study arm based on observed improvement in the pCR rate. This is because many other variables influence overall survival in addition to the achievement of pCR, including baseline prognosis, use and efficacy of endocrine therapy, and length of follow-up. These complexities are illustrated by the findings of the National Surgical Adjuvant Breast and Bowel Project (NSABP)-B27 study.23 The addition of docetaxel (3 times weekly for four cycles) to AC chemotherapy increased the pCR rate by 13% (breast only), but this did not translate to significantly improved 5-year survival for this arm (Table 25-1). Patients in either treatment arm who achieved pCR had excellent survival, but the B27 study was only powered to detect a difference in pCR rate between the treatments and was not powered to detect a survival difference.11 Furthermore, Table 25-1 illustrates how improvement in pCR observed in a neoadjuvant trial anticipated a survival difference in a phase III adjuvant trial for 3 recent treatment advances: addition of a taxane to anthracycline-based chemotherapy (white),11,23,24 more frequent paclitaxel dosing schedule (gray),25,26 and the addition of trastuzumab (Herceptin) to sequential anthracycline-taxane chemotherapy (blue).27-29 Therefore, neoadjuvant chemotherapy trials provide a valid clinical model in which to test for further improvements in adjuvant treatment.
| NEOADJUVANT TREATMENT | ADJUVANT TREATMENT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial | Treatment Arms | N | pCR (%) | P | Treatment Trial | Arms | N | 5-yr DFS | P (HR) |
| NSABP-B27 | AC + Tam | 802 | 13 | NSABP-B27 | AC + Tam | 802 | 67.7% | ||
| AC/Dx4 + Tam | 803 | 26 | <0.0001 | AC/Dx4 + Tam | 803 | 71.1% | 0.22 (0.90) | ||
| NSABP-B28 | AC + Tam | 1529 | 72% | ||||||
| AC/Tx4 + Tam | 1531 | 76% | 0.006 (0.83) | ||||||
| CALGB-9344 | AC | 1551 | 65% | ||||||
| AC/Tx4 | 1570 | 70% | 0.002 (0.83) | ||||||
| MDACC | 3-weekly Tx4/FAC | 131 | 16 | E1199 | 3-weekly Tx4/AC | 1253 | 65% | ||
| weekly Tx12/FAC | 127 | 28 | 0.02 | weekly Tx12/AC | 1231 | 70% | 0.006 (0.79) | ||
| MDACC | Tx4/FEC | 19 | 26 | N9831 + NSABP-B31 | AC/Tx4 | 1679 | 1253 | ||
| Tx4/FEC + Herceptin | 23 | 65 | 0.016 | AC/Tx4 + Herceptin | 1672 | 1231 | 0.0001 (0.48) | ||
| Tx4/FEC + Herceptin | 22 | 55 | |||||||
There is current debate about whether pathologic response from preoperative chemotherapy is a useful end point for hormone receptor (HR)-positive breast cancer. A potential disadvantage of relying solely on pCR is that the frequency of this outcome is comparatively low in HR-positive breast cancer, usually in the range of 3% to 9%.30,31 Indeed, HR-positive disease constitutes the majority of breast cancers, and the extent of receptor positivity can have confounding effects on results of chemotherapy trials.32 Nonetheless, it is clear that the incremental improvements in adjuvant chemotherapy (such as inclusion of anthracyclines, addition of taxanes, and dosing schedule of paclitaxel) do benefit patients with HR-positive breast cancer with acceptable reduction in the relative risk of disease recurrence in the range of 20% to 30%.31,33,34 Therefore, it is reasonable to conclude that less than complete remission from chemotherapy for HR-positive breast cancer can provide a survival benefit. It is possible that some degree of tumor response to adjuvant chemotherapy effectively reduces tumor burden and improves the duration of benefit from subsequent adjuvant endocrine therapy: in essence, partial efficacy from chemotherapy, followed by partial efficacy from endocrine therapy, to achieve full adjuvant efficacy. In that context, an end point of pCR would have a favorable prognosis, but the other category of RD would also include patients with an excellent prognosis, essentially blurring the distinction between categories of pathologic response.
The American Joint Committee on Cancer (AJCC) staging system for breast cancer was revised to designate with a “y” prefix when stage has been obtained from the pathologic evaluation of a surgical resection specimen that was obtained after a patient received neoadjuvant therapy.35 Of course, y-Stage 0 is identical to pCR. The main purpose of this is to distinguish between the prognosis of pathologic stage in breast cancer with and without prior systemic therapy. Two reports that evaluated AJCC y-Stage categories after neoadjuvant chemotherapy have both demonstrated a prognostic association.17,36 The pathologic stage of RD can also be combined with pretreatment tumor clinical and pathologic characteristics to define 5 categories of clinical-pathologic score (CPS) or also combined with estrogen receptor (ER) and grade (CPS+EG) to define 7 categories of response.37 This approach estimates the extent of down-staging after neoadjuvant treatment as well as biologic characteristics of the disease that are known to be associated with response to chemotherapy. It was developed from 932 patients at the MD Anderson Cancer Center (MDACC) who were variably treated. A potential advantage of this approach is that it enriched the proportion of patients in the good prognosis category, from 14% with pCR to 22% with CPS score 0 and 24% with CPS+EG score 0 or 1 (Fig. 25-1). However, relatively few patients were identified with high (more than 50%) risk of relapse following poor response to neoadjuvant therapy (3% with CPS score 4, and 6% with CPS+EG score 5 or 6). Those results might reflect the effects of adjuvant hormonal therapy in those with ER-positive disease and that many of the earlier patients in this cohort underwent surgery midway through their adjuvant chemotherapy regimen. The published work to date represents the development of an index, and therefore independent validation of this new method will be necessary. However, the method could become clinically useful as a nomogram to summarize information about tumor response that combines an estimate of tumor response with biologic features of the tumor.
Figure 25-1

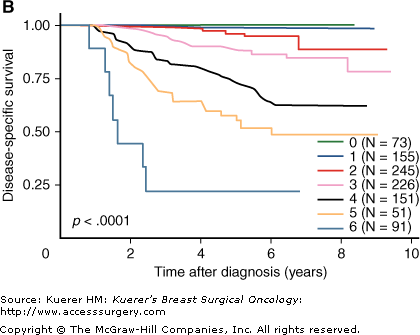
Disease-specific survival curves for categories of clinicopathologic score (CPS) alone (A), or combined with estrogen receptor status and grade as the CPS+estrogen grade (EG) score (B). (Reproduced, with permission, from Jeruss JS, Mittendorf EA, Tucker SL, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol. 2008;26:246-252.)
It appears that the prognostic relevance of a micrometastases in a regional lymph node after neoadjuvant chemotherapy is different from a micrometastasis when surgery is performed before chemotherapy, suggesting that any residual metastatic disease in the regional lymph nodes is important.38 However, those data were obtained from an era before sentinel lymph node (SLN) procedures became available, with more thorough gross and microscopic evaluation of those nodes. Therefore, the implication of those results should be interpreted with caution at this time. Other data from the era before the sentinel biopsy indicate that conversion from pathologic node-positive status at diagnosis (confirmed by needle biopsy at the time) to pathologic node-negative status after neoadjuvant chemotherapy is associated with an excellent survival that is significantly better than failure to down-stage from positive- to negative-node status.16 Those results, and the results described later, support an opinion that evaluation of nodal status after neoadjuvant treatment provides more prognostic information than nodal status before neoadjuvant treatment. It is therefore prudent to have a consistent approach to the timing of SLN biopsy for clinical trials of neoadjuvant chemotherapy that intend to use pathologic response, or survival, as an end point.
However, there is currently controversy about whether SLN sampling should be routinely performed to stage the axilla before neoadjuvant treatment or after the treatment has been completed. Is it more useful prognostic information to know the pathologic extent of nodal cancer burden before or after adjuvant chemotherapy? At MDACC we prefer posttreatment SLN evaluation because it reduces the number of surgeries and we believe it has stronger prognostic value, but there are clinical concerns about the accuracy of sampling at that time and the potential information for radiation therapy planning. The issue remains controversial and may be the subject of a prospective randomized trial.
Other changes occur in the tissues after neoadjuvant chemotherapy. The number of cancer cells within a tumor mass can decrease, and that reduction in cancer cellularity can be highly variable within a tumor mass. Decreased cancer cellularity is usually associated with fibrosis and some degree of chronic inflammatory infiltrate. Indeed, the Miller and Payne classification ignores tumor size and nodal status altogether, and it estimates only the decrease in cancer cellularity by comparing the initial diagnostic core biopsy with the residual cellularity in the tumor bed after treatment.10 The Miller and Payne classification includes 5 categories: grade 1, no reduction; grade 2, minor loss (less than 30%); grade 3, moderate loss (30%-90%); grade 4, marked loss (more than 90%); and grade 5, complete loss of cellularity. This was reported to be prognostic in 170 patients, with the largest separation in disease-free survival observed between grade 5 and the other grades.10 It has also been demonstrated that the reduction in cellularity is often greatest when the residual tumor is small, suggesting a relationship between residual size and cellularity.39 However, there was considerable variability in the extent of cytoreduction within any residual y-Stage category.39 This suggests that tumor shrinkage and cytoreduction are associated but not uniformly correlated. Although microscopic RD, altered cytologic appearance, and estimated tumor volume less than 1 cm3 also indicate good response, these tend to be descriptive parameters and are also difficult to apply to tumor beds with dispersed microscopic foci of carcinoma.3-6,9,40
RD after neoadjuvant treatment includes a broad range of actual responses from near pCR to frank resistance. We developed a method to measure RD by combining histopathologic components of RD (cellularity, overall diameter, and number and extent of nodal involvement) into a numerical index of residual cancer burden (RCB).36 A free Web site is available to calculate RCB from the histopathologic variables (http://www.mdanderson.org/breastcancer_RCB). A detailed description of the rationale and methods is included here. We developed the RCB index in 241 patients who received 6 months of neoadjuvant chemotherapy with T/FAC, and validated it in a separate cohort of 141 patients who received 3 months of preoperative chemotherapy with FAC followed by an additional 3 months of postoperative adjuvant chemotherapy (FAC in 129, other in 12).36 In the T/FAC cohort, RCB was independently prognostic for distant relapse-free survival in a multivariate model that included age, pretreatment clinical stage, HR status and hormonal therapy, and pathologic response (pCR versus RD) (hazard ratio, 2.50; confidence interval, 1.70 to 3.69; p < 0.001) (Fig. 25-2). The bias-adjusted C-index for RCB as a prognostic factor was 0.77. The generalizability of RCB for prognosis of distant relapse was confirmed in the FAC-treated validation cohort, with a C-index of 0.70.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree