With improved pediatric survival from serious underlying illnesses, greater use of invasive vascular procedures and devices, and a growing awareness that vascular events occur among the young, venous thromboembolism (VTE) increasingly is recognized as a critical pediatric concern. This review provides background on etiology and epidemiology in this disorder, followed by an indepth discussion of approaches to the clinical characterization, diagnostic evaluation, and management of pediatric VTE. Prognostic indicators and long-term outcomes are considered, with emphasis on available evidence underlying current knowledge and key questions for further investigation.
With improved pediatric survival from serious underlying illnesses, greater use of invasive vascular procedures and devices, and a growing (albeit still suboptimal) awareness that vascular events do occur among the young, venous thromboembolism (VTE) increasingly is recognized as a critical pediatric concern. The focus of this review is on providing background on etiology and epidemiology in this disorder, followed by an in-depth discussion of approaches to the clinical characterization, diagnostic evaluation, and management of pediatric VTE. Prognostic indicators and long-term outcomes are considered, with emphasis placed on available evidence underlying present knowledge and key questions for further investigation.
Characterization
VTE is classified clinically by various relevant descriptors, including first episode versus recurrent, symptomatic versus asymptomatic, acute versus chronic (a distinction that can be difficult at times), veno-occlusive versus nonocclusive, and idiopathic versus risk associated. This last category includes clinical prothrombotic risk factors (eg, exogenous estrogen administration, indwelling central venous catheter, and reduced mobility) and blood-based thrombophilic conditions (eg, transient or persistent antiphospholipid antibodies [APAs], acquired or congenital anticoagulant deficiencies, and factor V Leiden or prothrombin G20210A mutations); the latter are discussed in greater detail later. Because of the frequency of indwelling central venous catheters as a major clinical risk factor for VTE in children, VTE also may be classified as catheter-related thromboembolism (CRT) versus non-CRT. VTEs also are distinguished anatomically by vascular type (ie, venous versus arterial); vascular distribution (eg, distal lower extremity versus proximal lower extremity versus central or superficial versus deep vasculature); and organ system affected, if applicable (eg, cerebral sinovenous thrombosis [CSVT] or pulmonary embolism). The use of systematic nomenclature and precise descriptors for VTE assists in optimizing clinical care and in evaluating clinical research evidence in the field.
Epidemiology
Several years ago, registry data revealed an estimated cumulative incidence of 0.07 per 10,000 (5.3 per 10,000 hospitalizations) for extremity deep venous thrombosis (DVT) or pulmonary embolism (PE) among non-neonatal Canadian children and an incidence rate of 0.14 per 10,000 Dutch children per year for VTE in general. More recently, an evaluation of the National Hospital Discharge Survey and census data for VTE in the United States disclosed an overall incidence rate of 0.49 per 10,000 per year.
Epidemiologic data have revealed that the age distribution of the incidence rate for VTE in children is bimodal, with peak rates in the neontal period and adolescence. The Dutch registry, for example, indicated a VTE incidence rate of 14.5 per 10,000 per year in the neonatal period, approximately 100 times greater than the overall rate in childhood, whereas the VTE-specific incidence rate in the United States among adolescents 15 to 17 years of age was determined as 1.1 per 10,000 per year, a rate nearly threefold that observed overall in children.
Epidemiology
Several years ago, registry data revealed an estimated cumulative incidence of 0.07 per 10,000 (5.3 per 10,000 hospitalizations) for extremity deep venous thrombosis (DVT) or pulmonary embolism (PE) among non-neonatal Canadian children and an incidence rate of 0.14 per 10,000 Dutch children per year for VTE in general. More recently, an evaluation of the National Hospital Discharge Survey and census data for VTE in the United States disclosed an overall incidence rate of 0.49 per 10,000 per year.
Epidemiologic data have revealed that the age distribution of the incidence rate for VTE in children is bimodal, with peak rates in the neontal period and adolescence. The Dutch registry, for example, indicated a VTE incidence rate of 14.5 per 10,000 per year in the neonatal period, approximately 100 times greater than the overall rate in childhood, whereas the VTE-specific incidence rate in the United States among adolescents 15 to 17 years of age was determined as 1.1 per 10,000 per year, a rate nearly threefold that observed overall in children.
Etiology
The pathogenesis of VTE readily can be appreciated by considering the Virchow triad, consisting of venous stasis, endothelial damage, and the hypercoagulable state. In children, greater than 90% of VTEs are risk associated (compared with approximately 60% in adults), with risk factors often disclosed from more than one component of this triad. Specific examples of VTE risk factors in children are shown in ( Fig. 1 ). One of the most common clinical prothrombotic risk factors in childhood is an indwelling central venous catheter. More than 50% of cases of DVT in children and more than 80% of cases in newborns occur in association with central venous catheters. The presence of an indwelling central venous catheter, underlying malignancy or disorder for which bone marrow transplantation was undertaken, and congenital cardiac disease and its corrective surgery all were highly prevalent in the Canadian pediatric thrombosis registry, whereas underlying infectious illness and the presence of an indwelling central venous cathether were identified as pervasive clinical risk factors in a recent cohort study analysis from the United States. It is likely that differences in the composition of referral populations contribute strongly to differences in the composition of VTE etiologies across major pediatric thrombosis centers.
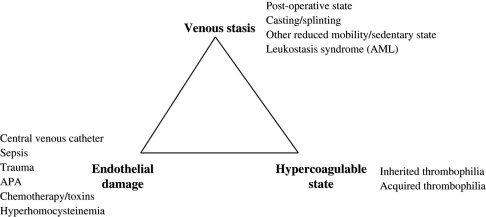
With regard to the third component of the Virchow triad, the hypercoagulable state, blood-based risk factors for VTE in children include inherited and acquired thrombophilic conditions and markers of coagulation activation (discussed later). Potent thrombophilic conditions (eg, APAs) in children frequently are acquired and, more rarely, may be congenital (eg, severe anticoagulant deficiencies). By contrast, mild congenital thrombophilia traits (eg, the factor V Leiden and prothrombin G20210A mutations) are common in white populations, with prevalences of approximately 5% and 2%, respectively. Thrombophilia potentially can be caused by any alteration in the hemostatic balance that increases thrombin production, enhances platelet activation or aggregation, mediates endothelial activation or damage, or inhibits fibrinolysis. Common examples of acquired thrombophilia in children include increased factor VIII activity with significant infection and inflammatory states, anticoagulant deficiencies resulting from consumption in bacterial sepsis and disseminated intravascular coagulation (DIC) or production of inhibitory antibodies in acute viral infection, and parainfectious development of APAs. To provide an appreciation of the magnitude of VTE risk increase associated with several congenital or genetically influenced thrombophilia traits, population-based VTE risk estimates derived from the adult literature are shown in Table 1 . As seen in Table 1 , the addition of standard-dose estrogen oral contraceptive pill to an underlying heterozygous factor V Leiden (in large part by virtue of a “double-hit” to the protein C pathway) substantially increases the risk for VTE from a baseline risk of 15 per 10,000 women in the United States, ages 15 to 17, per year to a risk of more than 500 per 10,000 (or 5%) per year.
| Trait/Condition | Venous Thromboembolism Risk Estimate (× Baseline) |
|---|---|
| Hyperhomocysteinemia | 2.5 |
| Prothrombin 20,210 mutation, heterozygous | 3 |
| Oral contraceptive pill (tandard dose estrogen) | 4 |
| Factor V Leiden mutation, heterozygous | 2–7 |
| OCP + factor V Leiden mutation, heterozygous | 35 |
| Factor V Leiden mutation, homozygous | 80 |
Clinical presentation
The degree of clinical suspicion for acute VTE in children should be influenced principally by (1) clinical prothrombotic risk factors and family history of early VTE or other vascular disease elicited on thorough interview; (2) known thrombophilia traits and risk factors; and (3) clinical signs and symptoms. The signs and symptoms of VTE depend on anatomic location and organ system affected and are influenced by characteristics of veno-occlusiveness and chronicity. The classic manifestation of acute extremity DVT is painful unilateral limb swelling. The lack of other physical examination findings (eg, Homans’ sign or presence of a palpable cord in the popliteal fossa) should not reduce the clinical index of suspicion of DVT. In upper extremity DVT with extension into, and occlusion of, the superior vena cava (SVC), signs and symptoms may include swelling of neck and face, bilateral periorbital edema, and headache. PE classically is manifest by sudden-onset, unexplained shortness of breath with pleuritic chest pain. When PE is proximal or extensive bilaterally in the distal pulmonary arterial tree, hypoxemia often is demonstrated. Associated right heart failure may manifest with hepatomegaly or peripheral edema. Proximal PE and especially saddle embolus can present with cyanosis or sudden collapse. In many cases, however, PE may be asymptomatic or produce only subtle symptoms in children, especially when involving limited segmental branches of the pulmonary arteries. In one retrospective series, only 50% of affected children had clinical symptoms attributable to PE. Acute CSVT may present with unusually severe and persistent headache, blurred vision, neurologic signs (eg, cranial nerve palsy and papilledema), or seizures. The classic findings in renal vein thrombosis (RVT) are hematuria and thrombocytopenia, sometimes associated with uremia (especially when bilateral). Presenting signs include oliguria (especially when bilateral) and, in the neonatal period (the time at which RVT is most common during childhood), a flank mass that often is palpable on examination. RVT in older children often is associated with nephrotic syndrome (a risk factor for VTE in general) and, hence, may present with associated stigmata of peripheral and periorbital edema when diagnosed at presentation of nephrosis. Thrombocytopenia may be a presenting manifestation not only of RVT but also of an intracardiac (eg, right atrial) thrombus, especially as in cases of CRT associated with sepsis and DIC. Portal vein thrombosis characteristically presents with splenomegaly and is associated with thrombocytopenia and, often, anemia; gastrointestinal bleeding at presentation typically signals the presence of gastroesophageal varices as a result of portal hypertension. Internal jugular vein thrombosis may manifest with neck pain or swelling and, in the Lemierre syndrome, also is associated classically with fever, trismus, and a palpable mass in the lateral triangle of the neck. Isolated intracardiac thrombosis in association with cardiac surgery or central venous catheter placement most often is asymptomatic.
Chronic VTE may be diagnosed incidentally without signs or symptoms (as sometimes occurs for CSVT during unrelated brain imaging) or, alternatively, may present with signs and symptoms of chronic venous obstruction or post-thrombotic syndrome (PTS) secondary to central venous or extremity thrombosis, including limb pain and edema, dilated superficial collateral veins, venous stasis dermatitis, or frank ulceration of the skin.
Diagnostic evaluation
Radiologic Imaging
Historically, venography has been the gold standard for diagnosis of venous thrombosis but limited by its invasiveness. In recent years, this modality has experienced a diminishing role with the development of effective noninvasive or minimally invasive radiologic imaging techniques. Radiologic imaging is used not only to confirm the clinical diagnosis of VTE but also to define the extent and occlusiveness of thrombosis. For suspected DVT of the distal or proximal lower extremity, compression ultrasonography with Doppler imaging typically is used for objective confirmation. When the thrombus may affect or extend into deep pelvic or abdominal veins, CT or MRI often is required. In suspected DVT of the upper extremity, compression ultrasound with Doppler effectively evaluates the limb, but other modalities (eg, echocardiography, CT, and MRI) are needed to disclose involvement of more central vasculature (eg, right atrial thrombosis and SVC thrombosis). In the case of asymptomatic nonocclusive extremity DVT, conventional venography may be used as an alternative to CT or MRI. To establish a diagnosis of DVT of the jugular venous system (such as in suspected cases of the Lemierre syndrome), compression ultrasound with Doppler imaging typically is used.
PE in children commonly is disclosed by spiral CT or, alternatively, ventilation-perfusion scan, the latter generally is suboptimal in cases wherein other lung pathology exists and at centers wherein availability of (and expertise with) this modality is limited. CSVT typically is diagnosed by standard CT or CT venography or, alternatively, MRI or MR venography. The diagnosis of RVT most often is made clinically in neonates and supported by Doppler ultrasound findings of intrarenal vascular resistive indices; however, in some cases a discrete thrombus may be suggested by Doppler ultrasound (especially when extending into the inferior vena cava [IVC]) or disclosed further via MR venography. When RVT occurs in older children, Doppler ultrasound or CT often is diagnostic. Similarly, portal vein thrombosis typically is visualized by Doppler ultrasound or CT.
When new-onset venous thrombosis is evaluated in patients in areas of anatomic abnormality of the venous system (eg, extensive collateral venous circulation due to a prior VTE episode, May-Thurner anomaly, or atretic IVC with azygous continuation), more sensitive methods, such as CT venography or magnetic resonance (MR) venography, often are required to delineate the vascular anatomy adequately and the presence, extent, and occlusiveness of thrombosis. In some cases, conventional venography may be required.
MR venography is more expensive than CT venography, typically requires sedation in children less than 8 years of age or those who are developmentally delayed or very anxious, and its feasibility during acute VTE evaluation may be limited by availability of MR-trained technologists. MR venography offers a significant advantage over CT venography, however, in that it provides diagnostic sensitivity at least as great as CT venography, without engendering the significant radiation exposure of the latter modality.
Laboratory Evaluation
Diagnostic laboratory evaluation for pediatric acute VTE includes a complete blood count, comprehensive thrombophilia evaluation (discussed previously), and beta-hCG testing in postmenarchal women. Additional laboratory studies may be warranted depending on associated medical conditions and VTE involvement of specific organ systems. Table 2 summarizes a panel of thrombophilia traits and markers identified as risk factors for VTE in pediatric studies and recommended by the Scientific and Standardization Committee Subcommitee on Perinatal and Pediatric Haemostasis of the International Society on Thrombosis and Haemostasis for the diagnostic laboratory evaluation of acute VTE in children. The panel is comprised of testing for states of anticoagulant (eg, protein C, protein S, and antithrombin) deficiency and procoagulant (eg, factor VIII) excess, mediators of hypercoagulablity or endothelial damage (eg, APAs, lipoprotein(a), and homocysteine), and markers of coagulation activation (eg, D-dimer).
| Condition/Marker | Testing Methods |
|---|---|
| Genetic | |
| Factor V Leiden polymorphism | PCR |
| Prothrombin G20210A polymorphism | PCR |
| Elevated plasma lipoprotein(a) concentration a | ELISA |
| Acquired or genetic | |
| Antithrombin deficiency | Chromogenic (functional) assay |
| Protein C deficiency | Chromogenic (functional) assay |
| Protein S deficiency | ELISA for free (ie, functionally active) protein S antigen |
| Elevated plasma factor VIII activity b | One-stage clotting assay (aPTT-based) |
| Hyperhomocysteinemia | Mass spectroscopy |
| APAs | ELISA for anticardiolipin and anti-β2-glycoprotein I IgG and IgM; clotting assay (dilute Russell viper venom time or aPTT-based phospholipid neutralization method) for LA |
| DIC | Includes platelet count, fibrinogen by clotting method (Clauss), and D-dimer by semiquantitative or quantitative immunoassay (eg, latex agglutination) |
| Activated protein C resistance | Clotting assay (aPTT based) |
a Although desginated here as genetic, lipoprotein(a) also may be elevated as part of the acute phase response.
b Noted as worthy of consideration in original International Society on Thrombosis and Haemostasis recommendations; this since has been shown a prognostic marker in pediatric thrombosis. Additional testing involving the fibrinolytic system and systemic inflammatory response also is noted as worthy of consideration.
Treatment
A summary of conventional antithrombotic agents and corresponding target anticoagulant levels, based on recent pediatric recommendations, is provided in Table 3 for initial (ie, acute phase) and extended (ie, subacute phase) treatment. Conventional anticoagulants attenuate hypercoagulability, decreasing the risk for thrombus progression and embolism, and rely on intrinsic fibrinolytic mechanisms to dissolve the thrombus over time. The conventional anticoagulants used most commonly in children include heparins and warfarin. Heparins, including unfractionated heparin (UFH) and low molecular weight heparin (LMWH), enhance the activity of antithrombin, an intrinsic anticoagulant protein that serves as a key inhibitor of thrombin. Warfarin acts through antagonism of vitamin K, thereby interfering with γ-carboxylation of the vitamin K–dependent procoagulant factors II, VII, IX, and X and intrinsic anticoagulant proteins C and S.
| Agents and Target Anticoagulant Activities | |||
|---|---|---|---|
| Episode | Initial Treatment | Extended Treatment | Duration of Therapy, by Etiology |
| First | UFH 0.3–0.7 anti-Xa U/mL | Warfarin INR 2.0–3.0 | Resolved risk factor: 3–6 months |
| LMWH 0.5–1.0 anti-Xa U/mL | LMWH 0.5–1.0 anti-Xa U/mL |
| |
| Recurrent | UFH 0.3–0.7 anti-Xa U/mL | Warfarin INR 2.0–3.0 | Resolved risk factor: 6–12 months |
| LMWH 0.5–1.0 anti-Xa U/mL | LMWH 0.5–1.0 anti-Xa U/mL |
| |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree