Childhood cancer survivors are at increased risk of serious morbidity, premature mortality, and diminished health status. Proactive and anticipatory risk-based health care of survivors and healthy lifestyles can reduce these risks. In this article, the authors first briefly discuss four common problems of survivors: neurocognitive dysfunction, cardiovascular disease, infertility and gonadal dysfunction, and psychosocial problems. Second, the authors discuss the concept of risk-based care, promote the use of recently developed evidence-based guidelines, describe current care in the United States, Canada, and the Netherlands, and articulate a model for shared survivor care that aims to optimize life long health of survivors and improve two-way communication between the cancer center and the primary care physician.
Cancer in childhood or adolescence is rare. Each year, for every 100,000 persons under the age of 21 years, 16 are diagnosed with cancer. Today, more than 80% of those diagnosed with a childhood cancer will become a long-term survivor. Many cancer survivors will develop serious morbidity, die at a young age from noncancer causes, and experience diminished health status. Among children treated in the 1970s to 1990s, about 75% will develop a chronic disease by 40 years of age, and over 40% will develop a serious health problem. The absolute excess risk of premature death from a second cancer, cardiovascular disease, or pulmonary disease is significantly elevated beyond 30 years after the cancer diagnosis. Almost half of long-term survivors will have moderate to extremely diminished health status, including limitations in activity and functional impairment. Although some serious problems occur during the cancer therapy or soon thereafter (long-term effects), the majority do not become clinically apparent until many years after the cancer has been cured (late effects). Contemporary therapy has evolved with a primary aim of not only improving cure but also decreasing the risk of long-term sequelae. It is anticipated that children treated in the 21 st century will not experience the frequency and severity of morbidity of those treated in the late 1900s. Furthermore, with proactive and anticipatory risk-based care and healthy lifestyles, the frequency and severity of many late effects of cancer therapy can be significantly reduced.
Most childhood cancer survivors in North America and Europe are not followed at a cancer center. Instead, over time, they generally drift back to the care of a primary care physician without a formal transition from the cancer center, generally unaware of their risks, without a summary of their cancer or cancer treatment, and with an inadequate understanding of their previous therapy. In a routine year in a typical primary care practice, a clinician is likely to see fewer than five childhood cancer survivors, each with a different cancer treated with a different regimen. Recognizing the competing demands of a busy primary care practice and the relative infrequency of seeing a childhood cancer survivor, it can be difficult to stay up-to-date with the health risks associated with different types of cancer therapy, much less with the recommendations for surveillance. However, the primary care physician can play a pivotal role in the health and well being of a childhood cancer survivor by delivering risk-based health care.
This article is intended to assist the primary care physician in this role. With a focus on contemporary therapy, the authors begin by providing a brief discussion of four major types of late effects about which survivors and their families commonly have questions, including neurocognitive dysfunction, cardiovascular disease, infertility and gonadal dysfunction, and psychosocial problems. While these questions are often directed to the oncologist during therapy, families may seek further input from their primary care physician. In the authors’ experience, these are also the most common questions that survivors or their families will ask years after the cancer therapy, often when they are no longer followed by the oncologist. Following these four topics, the authors discuss the concept of risk-based care, promote the use of recently developed evidence-based guidelines, describe current care in the United States, Canada, and the Netherlands, and articulate a model for shared survivor care that aims to optimize life-long health of survivors and improve two-way communication between the cancer center and the primary care physician. It is not the intent of this article to provide an exhaustive review of late effects, as recent publications have provided this information based upon treatment exposure or affected organ system. Two excellent books also provide much detail regarding late effects and the care of this population. Rather, the goal of this article is to orient the reader regarding the key problems, highlight ways that a primary care physician can positively influence the health of childhood cancer survivors, and point toward reliable resources for further inquiry.
Neurocognitive dysfunction
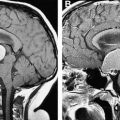
The potential for neurocognitive dysfunction is perhaps the most worrisome outcome to survivors and parents alike. When neurocognitive problems occur, children commonly present with school difficulties. Primary care physicians that deliver care for survivors should be aware of those at greatest risk, recognize the school difficulties associated with prior cancer therapy, and have an approach to screening, intervention and advocacy. Often this will involve helping the child and the family obtain the legally mandated supports required from the school system. Survivors of central nervous system (CNS) tumors and acute lymphoblastic leukemia (ALL) are at particular risk of neurocognitive late effects, but difficulties have been observed in patients treated with a stem cell transplant or with radiation for tumors of the head or neck. Cranial radiotherapy, particularly higher doses, is the major risk factor for adverse neurocognitive functioning, and survivors of CNS tumors treated with radiation at a young age are at considerable risk of global neurocognitive difficulties. Fortunately, neurocognitive dysfunction is much less common and severe with contemporary ALL therapy, where cranial radiotherapy is no longer used in patients at low or standard risk of CNS relapse. However, two-thirds of studies of children treated for ALL with chemotherapy alone demonstrate some degree of neurocognitive decline, with methotrexate, corticosteroids, and high-dose cytarabine most frequently associated with neurocognitive late effects. Female gender, younger age at therapy (particularly children less than 3 years), and increasing time from treatment increase the risk of these sequelae. Worsening academic performance is usually related to a reduced rate of skill acquisition rather than to loss of previously learned information, and is independent of the number of days of school missed because of therapy.
Survivors may have impairment in any area of neurocognitive function, but problems with attention and concentration, processing speed, visual perceptual skills, executive function, and memory are most common. Deficits in attention often manifest without hyperactivity, and can be misinterpreted as disinterest or bad behavior. Careless errors, incomplete assignments, and inconsistent academic performance are common, and these survivors often need extra time to complete their schoolwork. This can be compounded by difficulties with planning and organization. Mathematics, reading, and spelling are the most frequently impacted academic areas, and many survivors of ALL and CNS tumors require special education services. School difficulties may not manifest during the primary grades when rote-learning (memorization by repetition) may be relatively intact, but become evident as children transition to middle or high school where organizational, reasoning, and time management skills become essential to successful school performance.
When following a childhood cancer survivor, a primary care physician should assess school performance annually. Many pediatric cancer survivor programs obtain detailed neuropsychologic assessments in survivors at higher risk of difficulties, but in some circumstances these tests must be arranged by the survivor’s primary care physician. Unfortunately, many insurance providers do not cover this service, and test results may be available only from evaluations performed through the school system. While school-based testing can be helpful in developing an individualized education program, some important, subtle late effects may be missed. The primary care physician can assist parents by educating school personnel about the academic challenges faced by survivors. Several United States federal laws protect the rights of children with mental and physical limitations to receive special education, accommodation, and related services within the school system, and in many cases, these statutes can be applied to cancer survivors with learning difficulties.
Once completed, information from neuropsychologic assessments should be shared with the school. Simple educational accommodations include locating the child in the front of the classroom where there is less distraction, reducing the number of items on multiple choice tests, breaking assignments into discrete steps, and allowing more time for the completion of examinations. Other interventions, such as cognitive remediation and pharmacotherapy, are currently undergoing investigation with multicenter trials. Even if no specific educational intervention is identified after an initial assessment, it is important to continually reassess a survivor’s needs, because deficits may develop over time.
Cardiovascular disease
The developing cardiovascular system of a child or adolescent is very vulnerable to cancer therapy. A cardiomyopathy may develop following exposure to anthracyclines. Mantle radiotherapy promotes the development of coronary and carotid artery disease. In addition, perhaps most commonly, premature cardiovascular disease may result from alterations in multiple organ systems. The following sections describe each of these outcomes and emphasize the role of surveillance and prevention.
Anthracycline-Induced Cardiomyopathy
Anthracyclines, including doxorubicin and daunorubicin, are an important class of chemotherapeutic agents in the treatment of children with cancer. About half of those treated with contemporary therapy receive an anthracycline. Unfortunately, anthracycline cardiotoxicity is a major and generally unavoidable complication of childhood cancer therapy. The consequences of anthracycline cardiotoxicity for survivors are extensive. Late effects, resulting from myocardial damage, can manifest as left ventricular dysfunction, clinical heart failure, or as cardiac death. Anthracycline cardiotoxicity can be divided in asymptomatic (subclinical) and symptomatic (clinical) cardiotoxicity. Asymptomatic cardiotoxicity is defined as various cardiac abnormalities diagnosed with different diagnostic methods in asymptomatic patients; symptomatic cardiotoxicity is defined as clinical heart failure (CHF). Anthracycline-induced left ventricular dysfunction develops via two mechanisms: depressed contractility and an increased afterload. Late-onset anthracycline cardiotoxicity, occurring after the first year of survivorship, is the direct result of damage done during therapy, and is progressive.
Numerous studies have evaluated the cardiotoxic effects of anthracycline therapy in survivors of childhood cancer. As described in previous systematic reviews, some studies have methodologic limitations: only a selected subgroup of survivors have been evaluated, follow-up is incomplete, or nonstandardized diagnostic measurements have been used. For asymptomatic cardiotoxicity in childhood cancer survivors, a wide variation in the prevalence, from 0% to 56%, has been described. Differences in the selection of study groups, cumulative anthracycline dose, outcome definitions, and follow-up period could explain a part of this wide range. The risk of anthracycline-induced (A-) CHF in childhood cancer survivors has been evaluated in several cohort studies. In a cohort study of 831 subjects treated with anthracyclines for childhood cancer, the estimated risk of A-CHF, 20 years after the first dose of anthracyclines, was 9.8% for subjects who received a cumulative dose of greater than or equal to 300 mg/m 2 . Risk factors for anthracycline cardiotoxicity include higher cumulative dose of anthracyclines, radiotherapy involving the heart region, and a few studies suggest younger age at treatment and the female sex.
The risk of developing clinical heart failure for survivors treated with anthracyclines remains a life-long threat, and guidelines for long-term follow-up advise life-long cardiac monitoring for survivors treated with anthracycline. However, management of childhood cancer survivors with asymptomatic cardiotoxicity is unclear. Two studies have investigated the effect of angiotensin converting enzyme inhibitors in childhood cancer survivors. Although the results were promising, the noncontrolled trial suggested that enalapril treatment could delay, but not completely prevent, progression of subclinical and clinical cardiotoxicity in survivors. So primary prevention during treatment is essential, such as reducing the cumulative dose of anthracyclines, the use of possible less cardiotoxic anthracycline analogs, and reducing the peak dose or the use of cardioprotective agents.
Coronary and Carotid Artery Disease Following Mantle Radiotherapy
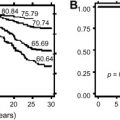
Moderate dose mantle irradiation (3,500 centigray or cGy–4,500 cGy) was the mainstay for treatment of early stage supradiaphragmatic Hodgkin’s disease from the 1960s to the 1980s. The mantle field encompasses the primary lymph node regions of the neck, supraclavicular, infraclavicular, axillary, and mediastinal areas. In a British cohort of 7,003 Hodgkin’s survivors with an average of 11.2 years of follow-up, the standardized mortality risk secondary to myocardial infarction was 3.2 for those who were treated with mantle irradiation. The absolute excess risk was 125.8 per 100,000 person-years. Aleman and colleagues reported that by 30 years after mediastinal irradiation, the cumulative incidence of myocardial infarction was 12.9%. They reported a standardized incidence ratio of 3.6 for myocardial infarction, with 357 excess cases per 100,000 person-years. Traditional risk factors (smoking, hypercholesterolemia, diabetes) increased risk. In Dutch Hodgkin’s survivors treated with moderate dose mediastinal irradiation (median, 3,720 cGy), Reinders and colleagues reported an actuarial risk of symptomatic ischemic coronary artery disease of 21.2% by 20 years after irradiation. This increased risk of premature coronary artery disease and myocardial infarction following mediastinal irradiation has been consistently reported in several other well-designed studies. Carotid artery disease has also been reported following mantle radiotherapy.
In the past 15 years, modified mantle radiotherapy with a lower total dose (2,000 cGy–2,500 cGy) to involved fields has been used in combination with multiagent therapy. More recent methods, of shielding the heart and equally weighting the anterior and posterior fields, appear to decrease the risk of cardiac disease. However, even with current shielding techniques, the proximal coronary arteries are within the modified mantle fields. So, despite modifications in therapy aimed at reducing risk, children and adolescents treated on contemporary Hodgkin’s disease protocols likely still face an increased risk of coronary and carotid artery disease. Longitudinal studies of survivors treated with contemporary therapy are needed to delineate the frequency, onset, and modifying factors of this risk. Aggressive risk reduction of traditional coronary artery disease risk factors (tobacco avoidance and cessation; optimum management of lipid disorders, diabetes mellitus, and hypertension; promotion of physical activity) should also reduce morbidity.
Cardiovascular Disease Following Childhood Acute Lymphoblastic Leukemia a Model for Multifactorial Cardiovascular Disease
Children who have survived ALL are more likely to be physically inactive and obese, have increased visceral adiposity, develop insulin resistance and dyslipidemia at a young age, and have poor cardiorespiratory fitness. These outcomes are in part related to cranial radiation, a therapy that is currently used in about 5% to 15% of children with ALL. However, children treated with chemotherapy alone also develop these outcomes, although the risk appears to be somewhat attenuated and possibly later in onset. This constellation of risk factors can be expected to lead to an increased incidence of cardiovascular disease, likely at a relatively young age. Similar outcomes have also been reported in brain tumor survivors and in those treated with a stem cell transplant. Research aimed at better understanding these relationships and the mechanisms leading to these outcomes is under way. In addition, the primary care physician should promote healthy behaviors (tobacco avoidance, healthy diet, and physical activity), screen for lipid disorders and insulin resistance, and closely monitor these survivors.
Cardiovascular disease
The developing cardiovascular system of a child or adolescent is very vulnerable to cancer therapy. A cardiomyopathy may develop following exposure to anthracyclines. Mantle radiotherapy promotes the development of coronary and carotid artery disease. In addition, perhaps most commonly, premature cardiovascular disease may result from alterations in multiple organ systems. The following sections describe each of these outcomes and emphasize the role of surveillance and prevention.
Anthracycline-Induced Cardiomyopathy
Anthracyclines, including doxorubicin and daunorubicin, are an important class of chemotherapeutic agents in the treatment of children with cancer. About half of those treated with contemporary therapy receive an anthracycline. Unfortunately, anthracycline cardiotoxicity is a major and generally unavoidable complication of childhood cancer therapy. The consequences of anthracycline cardiotoxicity for survivors are extensive. Late effects, resulting from myocardial damage, can manifest as left ventricular dysfunction, clinical heart failure, or as cardiac death. Anthracycline cardiotoxicity can be divided in asymptomatic (subclinical) and symptomatic (clinical) cardiotoxicity. Asymptomatic cardiotoxicity is defined as various cardiac abnormalities diagnosed with different diagnostic methods in asymptomatic patients; symptomatic cardiotoxicity is defined as clinical heart failure (CHF). Anthracycline-induced left ventricular dysfunction develops via two mechanisms: depressed contractility and an increased afterload. Late-onset anthracycline cardiotoxicity, occurring after the first year of survivorship, is the direct result of damage done during therapy, and is progressive.
Numerous studies have evaluated the cardiotoxic effects of anthracycline therapy in survivors of childhood cancer. As described in previous systematic reviews, some studies have methodologic limitations: only a selected subgroup of survivors have been evaluated, follow-up is incomplete, or nonstandardized diagnostic measurements have been used. For asymptomatic cardiotoxicity in childhood cancer survivors, a wide variation in the prevalence, from 0% to 56%, has been described. Differences in the selection of study groups, cumulative anthracycline dose, outcome definitions, and follow-up period could explain a part of this wide range. The risk of anthracycline-induced (A-) CHF in childhood cancer survivors has been evaluated in several cohort studies. In a cohort study of 831 subjects treated with anthracyclines for childhood cancer, the estimated risk of A-CHF, 20 years after the first dose of anthracyclines, was 9.8% for subjects who received a cumulative dose of greater than or equal to 300 mg/m 2 . Risk factors for anthracycline cardiotoxicity include higher cumulative dose of anthracyclines, radiotherapy involving the heart region, and a few studies suggest younger age at treatment and the female sex.
The risk of developing clinical heart failure for survivors treated with anthracyclines remains a life-long threat, and guidelines for long-term follow-up advise life-long cardiac monitoring for survivors treated with anthracycline. However, management of childhood cancer survivors with asymptomatic cardiotoxicity is unclear. Two studies have investigated the effect of angiotensin converting enzyme inhibitors in childhood cancer survivors. Although the results were promising, the noncontrolled trial suggested that enalapril treatment could delay, but not completely prevent, progression of subclinical and clinical cardiotoxicity in survivors. So primary prevention during treatment is essential, such as reducing the cumulative dose of anthracyclines, the use of possible less cardiotoxic anthracycline analogs, and reducing the peak dose or the use of cardioprotective agents.
Coronary and Carotid Artery Disease Following Mantle Radiotherapy
Moderate dose mantle irradiation (3,500 centigray or cGy–4,500 cGy) was the mainstay for treatment of early stage supradiaphragmatic Hodgkin’s disease from the 1960s to the 1980s. The mantle field encompasses the primary lymph node regions of the neck, supraclavicular, infraclavicular, axillary, and mediastinal areas. In a British cohort of 7,003 Hodgkin’s survivors with an average of 11.2 years of follow-up, the standardized mortality risk secondary to myocardial infarction was 3.2 for those who were treated with mantle irradiation. The absolute excess risk was 125.8 per 100,000 person-years. Aleman and colleagues reported that by 30 years after mediastinal irradiation, the cumulative incidence of myocardial infarction was 12.9%. They reported a standardized incidence ratio of 3.6 for myocardial infarction, with 357 excess cases per 100,000 person-years. Traditional risk factors (smoking, hypercholesterolemia, diabetes) increased risk. In Dutch Hodgkin’s survivors treated with moderate dose mediastinal irradiation (median, 3,720 cGy), Reinders and colleagues reported an actuarial risk of symptomatic ischemic coronary artery disease of 21.2% by 20 years after irradiation. This increased risk of premature coronary artery disease and myocardial infarction following mediastinal irradiation has been consistently reported in several other well-designed studies. Carotid artery disease has also been reported following mantle radiotherapy.
In the past 15 years, modified mantle radiotherapy with a lower total dose (2,000 cGy–2,500 cGy) to involved fields has been used in combination with multiagent therapy. More recent methods, of shielding the heart and equally weighting the anterior and posterior fields, appear to decrease the risk of cardiac disease. However, even with current shielding techniques, the proximal coronary arteries are within the modified mantle fields. So, despite modifications in therapy aimed at reducing risk, children and adolescents treated on contemporary Hodgkin’s disease protocols likely still face an increased risk of coronary and carotid artery disease. Longitudinal studies of survivors treated with contemporary therapy are needed to delineate the frequency, onset, and modifying factors of this risk. Aggressive risk reduction of traditional coronary artery disease risk factors (tobacco avoidance and cessation; optimum management of lipid disorders, diabetes mellitus, and hypertension; promotion of physical activity) should also reduce morbidity.
Cardiovascular Disease Following Childhood Acute Lymphoblastic Leukemia a Model for Multifactorial Cardiovascular Disease
Children who have survived ALL are more likely to be physically inactive and obese, have increased visceral adiposity, develop insulin resistance and dyslipidemia at a young age, and have poor cardiorespiratory fitness. These outcomes are in part related to cranial radiation, a therapy that is currently used in about 5% to 15% of children with ALL. However, children treated with chemotherapy alone also develop these outcomes, although the risk appears to be somewhat attenuated and possibly later in onset. This constellation of risk factors can be expected to lead to an increased incidence of cardiovascular disease, likely at a relatively young age. Similar outcomes have also been reported in brain tumor survivors and in those treated with a stem cell transplant. Research aimed at better understanding these relationships and the mechanisms leading to these outcomes is under way. In addition, the primary care physician should promote healthy behaviors (tobacco avoidance, healthy diet, and physical activity), screen for lipid disorders and insulin resistance, and closely monitor these survivors.
Fertility and gonadal dysfunction
When a child or adolescent is diagnosed with cancer, the discussion of cancer therapy is difficult and complicated, as the oncologist describes the response rates of various protocols, the associated acute toxicities of therapy, and the potential for future health problems related to the therapy. During this stress laden period when therapeutic decisions are made, as a parent faces the potential of losing a child, details regarding the potential for infertility and gonadal dysfunction are often not understood or remembered by families, and sometimes are not adequately provided by the cancer treating team. Later, as the cancer is cured and the interval from completion of the cancer therapy lengthens, questions regarding fertility and gonadal function become more prevalent. The loss of fertility (or even the fear of impaired fertility) and alterations in gonadal function influence the survivor’s developing body image, dating relationships, and marriage patterns.
Fertility is the most difficult outcome to study in survivors, as the primary endpoint is pregnancy, an outcome that is influenced by many physical and societal factors beyond the direct effect of the cancer therapy on ovarian or testicular function. Fertility is particularly difficult to study in males, as many men are not willing to have a semen analysis and self-reporting a successful impregnation is subject to both over- and under-reporting biases. Further compounding the investigation of fertility in both genders are the often overlapping effects of different cancer therapies on the reproductive system, and the sometimes late recovery of function. Recognizing the complexity of this subject, a detailed description is beyond the scope of this article. Following is a brief overview; for the clinician interested in better understanding these outcomes, two excellent articles written by leading researchers in this area are helpful resources.
Female Survivors, Acute Ovarian Failure, Premature Menopause, and Fertility Preservation
Though the ovaries during childhood and adolescence are relatively resistant to chemotherapy-induced damage, they are sensitive to radiation. Among 3,390 women in the Childhood Cancer Survivor Study (CCSS), loss of ovarian function during or shortly following completion of therapy (acute ovarian failure) was reported in 6.3%. More than 70% of women who had been treated with 2,000 cGy or more of ovarian irradiation had acute ovarian failure. Doses of ovarian irradiation below 1,000 cGy were capable of inducing acute ovarian failure in women who received concomitant alkylating agents (eg, cyclophosphamide) or were older at exposure. Survivors at greatest risk for acute ovarian failure are those treated with total body irradiation (TBI) in preparation for a stem cell transplant. Virtually all women treated with TBI after age 10 years will develop acute ovarian failure. In contrast, only 50% of those treated before 10 years of age will develop this outcome. In addition, women treated with high dose myeloablative therapy (eg, busulfan, melphalan, thiotepa), rather than TBI, before a stem cell transplant are at high risk of developing acute ovarian failure.
Female survivors who do not develop acute ovarian failure are potentially at risk of developing premature menopause (ie, menopause before age 40 years) and having reduced ovarian reserve. Among 2,819 women in the CCSS cohort who did not have acute ovarian failure, Sklar and colleagues reported a relative risk of nonsurgical premature menopause of 13.2, when compared with 1,065 siblings. Risk factors for premature menopause among survivors included older attained age, exposure to increasing dose of radiation to the ovaries, increasing dose of alkylating agents, and a diagnosis of Hodgkin’s disease. For women treated with an alkylating agent plus abdominopelvic radiation, the cumulative incidence of nonsurgical menopause approached 30% by 40 years of age.
In an assessment of 100 Danish childhood cancer survivors with a median age of 26 years, Larsen and colleagues reported that women with preserved menstrual cycles had sonographic and endocrine changes suggestive of diminished ovarian reserve. Decreased number of antral follicles per ovary was associated with treatment that included ovarian irradiation or use of alkylating agents, older age at diagnosis, and increasing years of therapy. With cranial radiation doses of 3,000 cGy or higher to the hypothalamic-pituitary axis, women may develop gonadotropin deficiency affecting fertility and sex hormone production. The consequences of ovarian failure and premature menopause extend beyond the issue of fertility and may include alterations in bone metabolism, leading to osteoporosis, sexual dysfunction, and body image changes.
In recent years, much attention has been directed toward preserving fertility in females undergoing cancer therapy during their childhood years. When radiation fields include the pelvis, the ovaries can be surgically transposed to a more protected location. However, even after transposition of the ovaries, some women will develop premature menopause secondary to their chemotherapy. Hormonal protection of the ovaries with a gonadotropin-releasing hormone analog has been attempted, with varying success, in small uncontrolled trials in patients undergoing therapy with moderate to high dose alkylating agents. Because the success rate of cryopreservation of unfertilized oocytes is very low, and the necessary ovarian hormonal stimulation before removal of the oocytes may delay cancer therapy, this approach is used infrequently in adolescents with cancer.
Lastly, ovarian tissue cryopreservation is an investigational method of fertility preservation that has the advantage of requiring neither a sperm donor nor ovarian stimulation. The American Society of Clinical Oncology recommends that oncologists discuss fertility preservation options as appropriate, and to refer interested patients and their families to reproductive specialists.
Encouragingly, women who become pregnant following childhood cancer generally have favorable outcomes. Among 1,953 women in the CCSS, 4,029 live births were reported and no association was found between chemotherapy and an adverse pregnancy outcome. Previous pelvic irradiation was associated with lower birth weight.
Male Survivors, Infertility, Fertility Preservation, and Androgen Deficiency
The germinal epithelium of the testis is sensitive to radiation. Even low-dose testicular irradiation is associated with decreased spermatogenesis, with doses above 200 cGy invariably causing oligospermia or azoospermia. Thus, males treated TBI, with a fractionated dose of 1,200 cGy to 1500 cGy, are often rendered infertile. Similarly, males with ALL who are treated with irradiation of the testis for a testicular relapse will almost always be azoospermic. Though the testes are shielded with modern techniques, scatter radiation from high-dose radiation can result in oligospermia or azoospermia. Examples include pelvic, inguinal, or spinal radiation for a sarcoma, Hodgkin’s disease, or CNS tumor, respectively. Lastly, radiation to the hypothalamic-pituitary axis may result in a gonadotropin deficiency, thus indirectly affecting spermatogenesis and reproductive potential.
Spermatogenesis is also quite affected by several chemotherapeutic drugs, including alkylating agents (eg, cyclophosphamide and ifosfamide), procarbazine, and cisplatin. Outcomes are agent specific and dose-dependent. Treatment with moderate to high-dose cyclophosphamide or ifosfamide often results in azoospermia. The combination of these two agents, used in the treatment of patients with Ewing sarcoma, causes infertility in virtually all males. Similarly, the combination of cisplatin with either ifosfamide or cyclophosphamide, used in the contemporary treatment of osteosarcoma, results in oligospermia or azoospermia in over 90% of males. High-dose melphalan or busulfan, used in some preconditioning regimens before a stem cell transplant, also causes impaired spermatogenesis in the vast majority of males. Early chemotherapeutic regimens used for Hodgkin’s disease, including six courses of mechlorethamine, vincristine, procarbazine, and prednisone, generally resulted in a high incidence of azoospermia. To preserve fertility, contemporary multimodality therapy of Hodgkin’s disease and non-Hodgkin lymphoma generally includes only three courses of an alkylating agent or procarbazine, alternating with another group of agents with a different set of toxicities.
Sperm cryopreservation is an effective method of fertility preservation in males. Unfortunately, spermarche does not occur until about 13 to 14 years of age, thus limiting sperm banking to adolescent males. In general, methods to preserve fertility in younger males, including testicular tissue cryopreservation, have not been successful. While it is recommended that the oncologist discuss sperm banking with all appropriate patients, it is also important for the primary care physician to be aware of this option if the patient or the family has any questions.
In comparison with the germinal epithelium, the Leydig cells are less affected by chemotherapy and radiotherapy. Testicular irradiation with doses of greater than 2,000 cGy and 3,000 cGy are associated with Leydig cell dysfunction in prepubertal and sexually mature males, respectively. Even with high dose cyclophosphamide, frankly subnormal levels of testosterone are rare, though Leydig cell dysfunction may be evidenced by an elevated luteinizing hormone level. Whether or not mild Leydig cell dysfunction will lead to premature androgen deficiency as this population ages is not known. Androgen deficiency can also result from hypogonadotropic hypogonadism following cranial radiotherapy.
Psychosocial issues in survivors and their families
The experience of being diagnosed and treated for cancer during childhood exerts considerable psychologic strain on both the patient and the family. Despite this, many survivors report normal psychologic health, and some even demonstrate psychologic growth as a result of their cancer experience. Additionally, most studies suggest that survivors are less likely to exhibit risky behaviors, such as cigarette smoking or drug use. However, on average, childhood cancer survivors are more likely to present with mental health disorders and to complain of chronic pain or fatigue than the general population. Hudson and colleagues reported that 17% of 9,535 young adult survivors of childhood cancer in the CCSS had depressive, somatic, or anxiety symptoms, and 10% reported moderate to extreme pain as a result of their cancer. Approximately one out five young adult survivors of childhood cancer reports symptoms of posttraumatic stress disorder (PTSD), characterized by re-experiencing elements of their prior cancer experience or its associated emotions, avoidance of people or places that remind them of their previous cancer, and increased anxiety or arousal. Avoidant behaviors may inhibit survivors from seeking appropriate follow-up care, particularly if care is delivered in the hospital where they received their cancer therapy. Additionally, both parents and siblings of survivors may develop symptoms of PTSD, and thus the primary care physician must extend their assessment of mental health to survivors’ families. Rather than developing PTSD, some survivors demonstrate posttraumatic growth and psychosocial thriving as a result of their cancer experience. Many rate themselves highly on their ability to cope as a result of their prior cancer, suggesting that this life-altering event promotes resiliency. When survivors do report psychologic distress, it is associated frequently with poorer health status, lower levels of income, and poorer social functioning.
Physicians need to be sensitive to the concerns expressed by survivors who often worry about fertility and parenthood, obtaining health and life insurance, educational difficulties, job availability after completing school, and their risk for future health problems, including second cancers. Although most survivors become socially independent and leave home at a similar age to the general population, rates of marriage are slightly lower. Survivors of CNS tumors are at particular risk of not marrying, and many are unable to live independently. Additionally, survivors of CNS tumors (but not other cancers) may be at increased risk of hospitalization for a psychiatric disorder. Primary care physicians can support these patients by providing interventions that improve health, support educational or occupational advancement to improve income potential, and promote social interaction. In particular, the development of a social network has been shown to enhance quality of life in survivors.
Several organizations provide services that can assist survivors, their parents, and their health care providers in dealing with the various challenges that may arise as these children and adolescents move beyond their primary cancer ( Table 1 ). Two books written for survivors or their families provide quality information and address the specific challenges of the cancer experience and survivorship.