Arterial ischemic stroke (AIS) is a rare disorder in children. Research suggests that risk factors, outcomes, and presentation are different from those of adult stroke. In particular, prothrombotic abnormalities and large vessel arteriopathies that are nonatherosclerotic seem to play a large role in the pathogenesis of childhood AIS. This review examines the epidemiology and etiologies of neonatal and childhood AIS and provides a detailed discussion of approaches to the clinical characterization, diagnostic evaluation, and management. Long-term outcomes of recurrent AIS and neuromotor, speech, cognitive, and behavioral deficits are considered. Emphasis is on evidence underlying current knowledge and key questions for further investigation.
Arterial ischemic stroke (AIS) is a rare, but increasingly recognized, disorder in children. Research in this area suggests that risk factors, outcomes, and even presentation are different from those of adult stroke. In particular, prothrombotic abnormalities and large vessel arteriopathies that are nonatherosclerotic seem to play a large role in the pathogenesis of childhood AIS. The purpose of this review is first to examine the epidemiology and etiologies of neonatal and childhood AIS and then provide a detailed discussion of approaches to the clinical characterization, diagnostic evaluation, and management of this disorder in pediatric patients. Long-term outcomes of recurrent AIS and neuromotor, speech, cognitive, and behavioral deficits are considered. Emphasis is on available evidence underlying current knowledge and key questions for further investigation.
Characterization
AIS is characterized by a clinical presentation consistent with stroke combined with radiographic evidence of ischemia or infarction in a known arterial distribution. Unless otherwise specified, throughout this article the term, “stroke,” is used synonymously with AIS. AIS in pediatrics is divided into two main categories: neonatal AIS and childhood (non-neonatal) AIS. Neonatal AIS is defined as any ischemic stroke occurring within the first 28 days of life and is subdivided further into prenatal, perinatal, and postnatal. Acute perinatal stroke usually presents with neonatal seizures during the first week of life. This differs from a presumed prenatal stroke, which typically presents at 4 to 8 months of life with an evolving hemiparesis. Because of the difficulty in determining the exact timing of presumed prenatal AIS, some investigators combine the two classifications as presumed prenatal/perinatal AIS. In contrast to neonatal stroke, childhood AIS typically presents with sudden onset of focal neurologic symptoms and signs and (given the broad differential diagnosis) requires an MRI of the brain with diffusion-weighted images to confirm the diagnosis of AIS (AIS presentation discussed later).
Epidemiology
The incidence of neonatal AIS recently is estimated at approximately 1 in 4000 live births annually. Similar estimates are provided by the United States National Hospital Discharge Survey, which recently published an incidence of 18 neonatal strokes per 100,000 births per year. Childhood AIS is less common than neonatal stroke, occurring one third to one tenth as often. The incidence of childhood stroke is approximately 2 to 8 in 100,000 per year in North America. Before the advent of MRI techniques, a Mayo Clinic retrospective analysis of cases in Rochester, Minnesota, in the late 1970s yielded an incidence of 2.5 strokes per 100,000 children per year. Although pre-MRI data likely underestimate the incidence of AIS because of the low sensitivity of CT for ischemia, more recent retrospective analyses using medical record review are subject to well-documented inaccuracies engendered by the International Statistical Classification of Diseases, 9th Revision coding. In addition, given the low index of suspicion for cerebrovascular events in children, the true incidence of pediatric AIS likely remains underdiagnosed.
Epidemiology
The incidence of neonatal AIS recently is estimated at approximately 1 in 4000 live births annually. Similar estimates are provided by the United States National Hospital Discharge Survey, which recently published an incidence of 18 neonatal strokes per 100,000 births per year. Childhood AIS is less common than neonatal stroke, occurring one third to one tenth as often. The incidence of childhood stroke is approximately 2 to 8 in 100,000 per year in North America. Before the advent of MRI techniques, a Mayo Clinic retrospective analysis of cases in Rochester, Minnesota, in the late 1970s yielded an incidence of 2.5 strokes per 100,000 children per year. Although pre-MRI data likely underestimate the incidence of AIS because of the low sensitivity of CT for ischemia, more recent retrospective analyses using medical record review are subject to well-documented inaccuracies engendered by the International Statistical Classification of Diseases, 9th Revision coding. In addition, given the low index of suspicion for cerebrovascular events in children, the true incidence of pediatric AIS likely remains underdiagnosed.
Etiology
The etiology of neonatal and childhood AIS is in many instances poorly understood, largely owing to the low incidence of the disease in the pediatric population and the lack of sufficient multicenter data on causal factors. The traditional ischemic stroke risk factors in adults, such as hypertension, atherosclerosis, diabetes, smoking, obesity, and hypercholesterolemia, are infrequent among neonates and older children who have AIS. In some instances of childhood AIS, such as congenital heart disease, sickle cell disease, and arterial dissection, the etiology readily is understood. For instance, with regard to cardiac risk factors, the Canadian Pediatric Stroke Registry found heart disease in 25% of patients who had pediatric AIS, whereas the prevalence of patent foramen ovale (PFO) in patients who had cryptogenic stroke was 40% to 50% compared with 10% to 27% in the general population. Some additional important risk factors for childhood AIS are identified, including vasculopathy, infection, head and neck trauma, previous transient ischemic attack (TIA) (defined as a focal neurologic deficit lasting less than 24 hours), and prothrombotic disorders. Often more than one risk factor is identified. Nevertheless, in the majority of childhood AIS cases, the etiology remains unclear, resulting in a broad subgroup of idiopathic childhood AIS.
Increasingly, arteriopathy (characterized by a disturbance of arterial blood flow within the vessel) is identified as a prevalent risk factor for childhood AIS, occurring in as many as 50% to 80% of cases. Often this arteriopathy is secondary to dissection of carotid or vertebral arteries, which accounts for 7% to 20% of all cases of childhood AIS, and may be caused by neck manipulation or head and neck trauma (although this association may be influenced by recall bias) and in rare cases may be related to underlying connective tissues disorders (eg, collagen defects). Nondissective arteriopathy also is related to certain infections. For example, there is a threefold increased risk for AIS within 1 year of acute varicella zoster virus (VZV) infection in childhood. Postvaricella arteriopathy typically exhibits a characteristic pattern of intracranial narrowing of the internal carotid artery (ICA), middle cerebral artery (MCA), and anterior cerebral artery (ACA), classically causing basal ganglia infarctions.
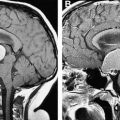
Increasingly, however, angiographic studies in childhood AIS identify MCA, ICA, and ACA arteriopathy without a known infectious or alternative cause; such cases consequently are characterized as idiopathic arteriopathies ( Fig. 1 ). A minority of these cases represent early moyamoya syndrome (defined as stenosis in the terminal portion of the ICAs bilaterally with the formation of tenuous collateral arteries, producing the classic angiographic “puff of smoke”) or possible moyamoya syndrome (recently characterized as unilateral stenosis in the terminal segment of an ICA with collaterals or the presence of bilateral stenosis of the terminal portion of the ICAs without collaterals) ( Fig. 2 ). Moyamoya, in turn, may be associated with sickle cell anemia, trisomy 21, a history of cranial irradiation for malignancy, or fibromuscular dysplasia; in many cases, however, moyamoya is of unclear etiology. In the majority of idiopathic arteriopathies, in which there is no progression to moyamoya, the phenomenon is termed, “transient cerebral arteriopathy,” in cases of a monophasic transient lesion that resolves or stabilizes within 6 months, and “chronic cerebral arteriopathy” in progressive cases. An elucidation of the etiology of these currently idiopathic arteriopathies will enhance the understanding of childhood stroke and may have an impact on future therapeutic management and outcomes in these patients.
Thrombophilia may contribute to AIS risk via arterial thrombosis or cerebral embolism of a venous thrombus through a cardiac lesion with right-to-left shunt. Thrombophilia risk factors in pediatric AIS include antiphospholipid antibodies, anticoagulant deficiencies, and hyperhomocysteinemia. Protein C is the most commonly associated anticoagulant deficiency, although in cases of AIS (and venous thromboembolism [VTE]) after acute varicella infection, antibody-mediated acquired protein S deficiency seems prevalent. It is likely that anticoagulant deficiencies are acquired most commonly secondary to viral-mediated inflammation, as is the case in varicella. As in VTE, however, severe congenital anticoagulant deficiencies also may be contributory. Although homozygosity for factor V Leiden or prothrombin G20210A polymorphism is a strong risk factor for thrombotic events, it remains unclear whether or not the factor V Leiden or prothrombin G20210A variant in heterozygous form confers a meaningful increase in the risk for pediatric AIS. Greatly elevated homocysteine levels classically are associated with metabolic disorders, such as homocysteinuria (resulting from cystathionine β-synthase deficiency), and mild to moderately elevated levels also occur in homozygous carriers of the methylenetetraydrofolate reductase mutation (MTHFR C677T). Among heterozgyotes, the latter cause of hyperhomocysteinemia is evident particularly in countries where routine folate supplementation of the diet is not undertaken. Another prothrombotic trait, elevated lipoprotein(a) concentration, is associated with increased odds of otherwise idiopathic (ie, apart from thrombophilia) childhood AIS. Other suspected blood-based risk factors for pediatric AIS, supported thus far by evidence from case series or case-control studies, include iron deficiency anemia, polycythemia, and thrombocytosis.
Given that focal arteriopathy is an uncommon finding in neonatal AIS, stroke etiology is less clear for this group than for childhood AIS. Nevertheless, congenital cardiac anomalies are established risk factors, and thrombophilia investigation has provided additional meaningful contributions. Similar thrombophilic risk factors are identified for neonatal AIS as for childhood stroke, including factor V Leiden, protein C deficiency, and elevated lipoprotein(a). The possibility of vertical transmission of prothrombotic molecular entities or vasoconstrictive agents (eg, antiphospholipid antibodies or cocaine, respectively) also is important to consider in the etiology of neonatal AIS but has not been studied systematically. In addition, maternal pregestational and gestational factors, such as infertility, placental infection, premature rupture of membranes, and preeclampsia, in the past few years have been found independently associated with neonatal AIS. Recent birth registry data are further suggestive of maternal vascular disease risk factors contributing to neonatal AIS, given the finding of an association with the development of seizures in term neonates (a common manifestation of neonatal AIS); however, further work is necessary to establish this potential risk factor. In addition, emerging data suggest a combination of maternal- and fetal-specific molecular risk factors in the development of placental pathology may be worthy of additional study regarding perinatal stroke risk.
Clinical presentation
The clinical presentation of AIS differs greatly among presumed prenatal, perinatal, postnatal, and childhood stroke. Within each classification, further variance of presentation exists and depends largely on the territory, extent, and timing of ischemia. The most common presentation for presumed prenatal stroke is evolving hemiparesis at 4 to 8 months of life. Typically, the hand is more involved than the arm, because of the high incidence of MCA distribution infarction, and the left hemisphere is affected more commonly than the right. Perinatal AIS has a similar predilection for the left MCA but often presents with focal neonatal seizures during the first week of life. Postnatal stroke may present similarly to perinatal AIS; in other instances, it presentation is like that of childhood stroke (discussed later).
The clinical presentation of childhood AIS is less stereotypical than that of pre- and perinatal AIS and is more dependent on the territory of ischemia. As a general rule, patients who have small- to medium-sized events present with sudden-onset focal neurologic deficits (such as hemiparesis, visual field deficits, aphasia, cranial nerve palsies, dyspahagia, and unilateral ataxia) without major alterations of consciousness. Focal neurologic deficit in the presence of preserved consciousness can aid in the diagnosis, as many more common pediatric illnesses, such as complex partial and generalized seizures or encephalitis, often have alteration of awareness. Larger strokes tend to have multiple deficits and alteration of consciousness. As discussed previously, a history of head or neck trauma, recent varicella infection, and the presence of sickle cell disease or cardiac disease may aid in understanding the underlying etiology. A recent single-center retrospective series of AIS in non-neonatal children found that a nonabrupt pattern of neurologic symptoms or signs (including those in whom the maximum severity of symptoms or signs developed more than 30 minutes from the time of symptom onset, the presentation of symptoms or signs was waxing and waning, or the presentation was preceded by recurrent transient symptoms or signs with intercurrent resolution) often was associated with findings of arteriopathy on diagnostic neuroimaging.
Strokes of metabolic etiology frequently manifest a progressive course of stroke-like episodes. Often, there is a family history of early-onset strokes, early-onset dementia, or severe migraines. A classic example of a metabolic stroke occurs in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). MELAS is caused by mitochondrial DNA defects encoding tRNA involved in the generation of ATP and the majority of cases (80%) have the mt3243 mutation. In addition to a maternal history of migraine and stroke, there often is a history of short stature, diabetes, hearing loss, occipital lobe seizures, and optic atrophy. The presentation often is characterized by stroke-like episodes with complete resolution of neurologic function. Over time, however, neurologic deficits persist, often in the form of hemianopia. Acute lesions observed by MRI can be distinguished from typical AIS by virtue of their paradoxic bright appearance on apparent diffusion coefficient (ADC) maps. In cases of a family history of cryptogenic stroke or atypical presentation of stroke, other inherited disorders (eg, non-MELAS mitochondrial DNA defects, organic acidemias, lysosomal diseases, severe congenital thrombophilias, and cerebral autosomal dominant arteriopathy with subcortical infarctions and leukoencephalopathy [CADASIL]), should be considered.
Diagnostic evaluation
Diagnostic evaluation of pediatric AIS is more extensive than in adult stroke because of the broad differential diagnosis at presentation and the higher incidence of ateriopathies in childhood AIS. In a child presenting with an acute focal neurologic deficit, multiple alternative etiologies must be considered, including hypoglycemia, prolonged focal seizures, prolonged postictal paresis (Todd’s paralysis), acute disseminated encephalomyelitis, meningitis, encephalitis, and brain abscess. For this reason, MRI brain with diffusion-weighted images is becoming the radiographic modality of choice in most cases of childhood AIS. Acutely, CT can rule out hemorrhage, tumor, and abscess and may be an appropriate first-line evaluation in some cases. Typically, this needs to be followed by an MRI with diffusion-weighted images to assess for cytotoxic edema, the hallmark of acute ischemia (see Fig. 1 ). The use of perfusion-weighted imaging largely is experimental in children but as acute interventions become more available in childhood AIS, this modality likely will be used increasingly as a means by which to identify potentially preservable territories of at-risk functioning brain. Furthermore, the pattern of infarction also may be suggestive of etiology. For example, a case of multiple infarctions in separate arterial distributions likely is thromboembolic, findings of occipital and parietal strokes that cross vascular territories may suggest MELAS, a distribution between vascular territories is consistent with watershed infarction suggestive of a hypotensive etiology, and a pattern of small multifocal lesions at the gray-white junction is suspicious for vasculitis.
Although routine CT and MRI evaluate for ischemia, hemorrhage, mass/mass effect, and other non-AIS pathologies, vascular imaging (magnetic resonance angiography [MRA], CT angiography [CTA], or conventional angiography) can demonstrate arteriopathy, including dissection, stenosis, irregular contour, or intra-arterial thrombosis of the head and neck. Typically, MRA or CTA is the modality used as first-line arterial imaging, unless MRI reveals a pattern consistent with small vessel vasculitis, in which case conventional angiography is indicated. If MRA or CTA suggests moyamoya or atypical vasculature, conventional angiography is warranted, as MRA or CTA may underestimate or overestimate the degree of disease.
An additional important component of diagnostic imaging in pediatric AIS is echocardiography with peripheral venous saline injection. In addition to disclosing a septal defect and other congenital cardiac anomalies, echocardiography with saline injection may disclose a small lesion, including a PFO that otherwise may not be detected by conventional transthoracic echocardiography. The prevalence of PFO in patients who have cryptogenic stroke is 40% to 50% compared with 10% to 27% in the general population. The use of Doppler imaging during echocardiography assists in determining the direction of shunt through a lesion, although the prognostic significance of the direction of shunt is not well established in pediatric stroke.
At a minimum, diagnostic laboratory evaluation in pediatric acute AIS involves a complete blood count, toxicology screen, complete metabolic panel, erythrocyte sedimentation rate/C-reactive protein (ESR/CRP) to assess for biochemical evidence of systemic inflammation that may suggest vasculitis or infection in the etiology of AIS, β-hCG testing in postmenarchal women, fasting lipid profile, and a comprehensive thrombophilia panel ( Box 1 ). Further investigation into metabolic, genetic, infectious, or rheumatologic diseases should be considered in cases of atypical presentation. In the setting of arteritis, arthritis, or elevated ESR/CRP, rheumatologic evaluation should be considered and include testing of antinuclear antibodies and rheumatoid factor. In childhood AIS with encephalopathy of unclear etiology, nonarterial distribution, or other multisystem disorders of unclear etiology (eg, hearing loss, myopathy, or endocrinopathy), testing should include lactate and pyruvate to screen for metabolic disorders and mitochondrial DNA testing for MELAS and related disorders. A lumbar puncture is indicated if infectious signs and symptoms are present and in other inflammatory states of unclear etiology. If cerebrospinal fluid (CSF) abnormalities are present, further infectious work-up and routine chemistries and blood counts are warranted. It may be prudent to test routinely for VZV, herpes simplex virus (HSV), enterovirus, and other known viral etiologies in the CSF when a lumbar puncture is performed in addition to performing routine bacterial cultures.