Immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by a low circulating platelet count caused by destruction of antibody-sensitized platelets in the reticuloendothelial system. ITP can be classified as childhood versus adult, acute versus chronic, and primary versus secondary. Persistence of thrombocytopenia defines the chronic form of the disorder. Secondary causes of ITP include collagen vascular disorders, immune deficiencies, and some chronic infections. This review focuses on the diagnosis and management of children who have acute and chronic ITP. Emphasis is placed on areas of controversy and new therapies.
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by a low circulating platelet count caused by destruction of antibody-sensitized platelets in the reticuloendothelial system. ITP can be classified based on patient age (childhood versus adult), duration of illness (acute versus chronic), and presence of an underlying disorder (primary versus secondary). Persistence of thrombocytopenia, generally defined as a platelet count of less than 150 × 10 9 /L for longer than 6 months, defines the chronic form of the disorder. Secondary causes of ITP include collagen vascular disorders, such as systemic lupus erythematosus (SLE); immune deficiencies, such as common variable immunodeficiency (CVID); and some chronic infections (eg, HIV and hepatitis C).
This article focuses on the diagnosis and management of children (under 18 years of age) who have acute and chronic ITP. Emphasis is placed on areas of controversy and new therapies.
Pathophysiology
The pathophysiology of ITP increasingly is understood better (reviewed by Cines and Blanchette. ) Not surprisingly, it is complex with involvement of many players in the human immune orchestra, including antibodies, cytokines, antigen-presenting cells, costimulatory molecules, and T and B lymphocytes (including T-helper, T-cytotoxic, and T-regulatory lymphocytes). Current knowledge is summarized later.
A key element in the pathophysiology of ITP is loss of self tolerance leading to the production of autoantibodies directed against platelet antigens. Evidence for an “antiplatelet factor” in the plasma of subjects who have ITP was provided in a seminal report from Harrington and coworkers in 1951. The investigators demonstrated that the infusion of plasma from subjects who had ITP into volunteers induced a rapid fall in platelet count and a clinical picture that mimics ITP. The “antiplatelet factor” subsequently was confirmed as an immunoglobulin. Now it is known that the autoantibodies in patients who have ITP mostly are of the IgG class with specificity against platelet-specific antigens, in particular, glycoproteins IIb/IIIa and Ib/IX. Unfortunately, accurate detection of platelet autoantibodies is difficult and not available routinely in most clinical hematology laboratories; clinicians should be aware that indirect platelet autoantibody tests (tests that detect free autoantibodies in the plasma) are inferior to direct tests (tests that detect platelet-bound autoantibodies) and that even with the best direct tests performed in expert immunohematology laboratories, the positivity rate in patients who have well-characterized ITP does not exceed 80%. A negative platelet antibody test, therefore, does not exclude a diagnosis of ITP. For this reason, platelet antibody testing is not recommended as part of the routine diagnostic strategy.
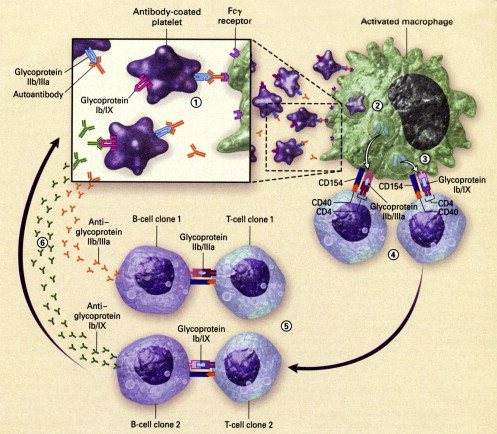
It is increasingly clear that cellular immune mechanisms play a pivotal role in ITP. The production of antiplatelet antibodies by B cells requires antigen-specific, CD4-postive, T-cell help ( Fig. 1 ). It also is possible that in some ITP cases, cytotoxic T cells play a role in the destruction of platelets. A possible sequence of events in ITP is as follows. A trigger, possibly an infection or toxin, leads to the formation of antibodies/immune complexes that attach to platelets. Antibody-coated platelets then bind to antigen-presenting cells (macrophages or dendritic cells) through low-affinity Fcγ receptors (Fcγ RIIA/Fcγ RIIIA) and are internalized and degraded. Activated antigen-presenting cells then expose novel peptides on the cell surface and with costimulatory help facilitate the proliferation of platelet antigen-specific, CD4-positive, T-cell clones. These T-cell clones drive autoantibody production by platelet antigen-specific B-cell clones. As part of the platelet destructive process in ITP, cryptic epitopes from platelet antigens are exposed, leading to the formation of secondary platelet antigen-specific T-cell clones, with stimulation of new platelet antigen-specific B-cell clones and broadening of the immune response. The autoantibody profile of individual patients who have ITP reflects activity of polyclonal autoreactive B-cell clones derived by antigen-driven affinity selection and somatic mutation.
Although increased platelet destruction clearly plays a key role in the pathogenesis of ITP, it is now recognized that impaired platelet production also is important in many cases. In adults, as many as 40% of ITP cases may have reduced platelet turnover, reflecting the inhibitory effect of platelet autoantibodies on megakaryopoiesis. Studies of platelet kinetics in children who have ITP are limited but it is possible that a similar situation exists. There also is evidence that platelet autoantibodies may induce thrombocytopenia by inhibiting proplatelet formation. Circulating thrombopoietin (TPO) levels in patients who have ITP typically are normal or increased only slightly, reflecting the normal or only slightly reduced TPO receptor mass in this acquired platelet disorder. In contrast, TPO levels are high in inherited platelet production disorders, such as thrombocytopenia-absent radii or congenital amegakaryocytic thrombocytopenia. TPO testing generally is not available, but these observations have led to the question of whether or not TPO or molecules mimicking TPO may increase platelet production and be a new treatment strategy in ITP. Several such agents currently are in clinical trials.
Differential diagnosis
Primary ITP is a diagnosis of exclusion. The question, “When does a low platelet count not mean ITP?” is important, especially for atypical cases. When an unexpected low platelet count in a child is obtained, artifact or laboratory error should be considered first and excluded. Pseudothrombocytopenia is an example of spurious thrombocytopenia that is caused by platelet aggregation and clumping in the presence of ethylenediamine tetraacetic acid (EDTA) anticoagulant. Examination of well-stained blood smears prepared from a venous blood sample collected separately into EDTA and 3.8% sodium citrate anticoagulant usually confirms or excludes pseudothrombocytopenia. A smear prepared from the collection tube with EDTA should demonstrate platelet clumping, whereas a smear prepared from the tube with sodium citrate should not. Some patients, however, have platelets that also clump in citrate anticoagulant.
A detailed history, careful physical examination, and results of selected tests confirm or eliminate common causes of secondary thrombocytopenia, such as SLE. A positive antinuclear antibody is common in children who have ITP and, as an isolated finding, does not confirm or exclude SLE; more specific tests, such as an anti–double-stranded DNA test, should be ordered if a diagnosis of SLE-associated ITP is suspected. A transfusion history should be obtained in all cases and, depending on the age of the child, the history should include questioning about drug use (prescription and nonprescription) and sexual activity. If relevant, testing for antibodies to hepatitis C and HIV should be performed.
A detailed family history should be obtained in all cases. Especially in children who have apparent “chronic” ITP and isolated moderate thrombocytopenia, the possibility of an inherited thrombocytopenia should be considered. The topic, “inherited thrombocytopenia: when a low platelet count does not mean ITP,” is the focus of an excellent review. The inherited thrombocytopenias can be classified based on platelet size (large, normal, and small) and gene mutations. They include conditions, such as the MYH9-related macrothrombocytopenias, Wiskott-Aldrich syndrome (WAS), and rare conditions, such as gray platelet syndrome ( Box 1 ). The pattern of inheritance (eg, X-linked in boys who have WAS) and abnormalities on peripheral blood smear (eg, Döhle-like inclusions in neutrophils of patients who have MYH9 disorders or pale agranular platelets in gray platelet syndrome) may provide important clues to the underlying disorder. Failure of patients who have apparent “chronic ITP” and moderate thrombocytopenia to respond to front-line platelet-enhancing therapies, such as high-dose intravenous (IV) immunoglobulin G (IVIG) or IV anti-D, should prompt consideration of an alternate diagnosis. Additional investigation in such cases should include screening for type 2B von Willebrand disease, pseudo–von Willebrand disease, and Bernard-Soulier syndrome. In males who have small platelets, WAS or X-linked thrombocytopenia should be considered. These latter conditions can be confirmed by screening for mutations in the WASP gene. Boys who have WASP gene mutations may have significant immunologic abnormalities.
Small platelets [MPV < 7 fL]
WAS
X-linked thrombocytopenia
Normal-sized platelets [MPV 7–11 fL]
Thrombocytopenia-absent radii
Congenital amegakaryocytic thrombocytopenia
Radioulnar synostosis and amegakaryocytic thrombocytopenia
Familial platelet disorder with associated myeloid malignancy
Large/giant platelets [MPV > 11 fL]
MYH9 a syndromes
- •
May-Hegglin anomaly
- •
Fechtner syndrome
- •
Epstein syndrome
- •
Sebastian syndrome
- •
Mediterranean thrombocytopenia
Bernard-Soulier syndrome
Velocardiofacial/DiGeorge syndrome
Paris-Trousseau thrombocytopenia/Jacobsen syndrome
Gray platelet syndrome
Abbreviation: MPV, mean platelet volume.
a MYH9 gene encodes for the nonmuscle myosin heavy-chain IIA.
Data from Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood 2004;103:390–8.
Differential diagnosis
Primary ITP is a diagnosis of exclusion. The question, “When does a low platelet count not mean ITP?” is important, especially for atypical cases. When an unexpected low platelet count in a child is obtained, artifact or laboratory error should be considered first and excluded. Pseudothrombocytopenia is an example of spurious thrombocytopenia that is caused by platelet aggregation and clumping in the presence of ethylenediamine tetraacetic acid (EDTA) anticoagulant. Examination of well-stained blood smears prepared from a venous blood sample collected separately into EDTA and 3.8% sodium citrate anticoagulant usually confirms or excludes pseudothrombocytopenia. A smear prepared from the collection tube with EDTA should demonstrate platelet clumping, whereas a smear prepared from the tube with sodium citrate should not. Some patients, however, have platelets that also clump in citrate anticoagulant.
A detailed history, careful physical examination, and results of selected tests confirm or eliminate common causes of secondary thrombocytopenia, such as SLE. A positive antinuclear antibody is common in children who have ITP and, as an isolated finding, does not confirm or exclude SLE; more specific tests, such as an anti–double-stranded DNA test, should be ordered if a diagnosis of SLE-associated ITP is suspected. A transfusion history should be obtained in all cases and, depending on the age of the child, the history should include questioning about drug use (prescription and nonprescription) and sexual activity. If relevant, testing for antibodies to hepatitis C and HIV should be performed.
A detailed family history should be obtained in all cases. Especially in children who have apparent “chronic” ITP and isolated moderate thrombocytopenia, the possibility of an inherited thrombocytopenia should be considered. The topic, “inherited thrombocytopenia: when a low platelet count does not mean ITP,” is the focus of an excellent review. The inherited thrombocytopenias can be classified based on platelet size (large, normal, and small) and gene mutations. They include conditions, such as the MYH9-related macrothrombocytopenias, Wiskott-Aldrich syndrome (WAS), and rare conditions, such as gray platelet syndrome ( Box 1 ). The pattern of inheritance (eg, X-linked in boys who have WAS) and abnormalities on peripheral blood smear (eg, Döhle-like inclusions in neutrophils of patients who have MYH9 disorders or pale agranular platelets in gray platelet syndrome) may provide important clues to the underlying disorder. Failure of patients who have apparent “chronic ITP” and moderate thrombocytopenia to respond to front-line platelet-enhancing therapies, such as high-dose intravenous (IV) immunoglobulin G (IVIG) or IV anti-D, should prompt consideration of an alternate diagnosis. Additional investigation in such cases should include screening for type 2B von Willebrand disease, pseudo–von Willebrand disease, and Bernard-Soulier syndrome. In males who have small platelets, WAS or X-linked thrombocytopenia should be considered. These latter conditions can be confirmed by screening for mutations in the WASP gene. Boys who have WASP gene mutations may have significant immunologic abnormalities.
Small platelets [MPV < 7 fL]
WAS
X-linked thrombocytopenia
Normal-sized platelets [MPV 7–11 fL]
Thrombocytopenia-absent radii
Congenital amegakaryocytic thrombocytopenia
Radioulnar synostosis and amegakaryocytic thrombocytopenia
Familial platelet disorder with associated myeloid malignancy
Large/giant platelets [MPV > 11 fL]
MYH9 a syndromes
- •
May-Hegglin anomaly
- •
Fechtner syndrome
- •
Epstein syndrome
- •
Sebastian syndrome
- •
Mediterranean thrombocytopenia
Bernard-Soulier syndrome
Velocardiofacial/DiGeorge syndrome
Paris-Trousseau thrombocytopenia/Jacobsen syndrome
Gray platelet syndrome
Abbreviation: MPV, mean platelet volume.
a MYH9 gene encodes for the nonmuscle myosin heavy-chain IIA.
Data from Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood 2004;103:390–8.
Childhood acute immune thrombocytopenic purpura
Clinical and Laboratory Features
Thrombocytopenia for less than 6 months defines the entity acute ITP. Typically, children who have acute ITP are young, of previous good health, and present with sudden onset of bruising or a petechial rash. In a series of 2031 children who had newly diagnosed ITP, reported by Kühne and colleagues in 2001 for the Intercontinental Childhood ITP Study Group (ICIS), the mean age at presentation was 5.7 years. Approximately 70% of the cohort were children ages 1 to 10 years with 10% of the cohort infants (older than 3 and less than 12 months old) and the remainder 20% older children (ages 10 to 16 years). Male and female children were affected approximately equally with the caveat that boys outnumbered girls in young children, especially those less than 1 year of age ( Fig. 2 ). The predominance of boys who had ITP in children under 10 years of age is reported in several other studies. In approximately two thirds of cases, the onset of acute ITP is preceded by an infectious illness, most often an upper respiratory tract infection; in a minority of cases, ITP follows a specific viral illness (rubella, varicella, mumps, rubeola, or infectious mononucleosis) or immunization with a live virus vaccine. The risk for ITP after mumps-measles-rubella vaccine is estimated at approximately 1 in 25,000 doses. In children who have acute ITP, the interval between the preceding infection and the onset of purpura varies from a few days to several weeks, with the most frequent interval approximately 2 weeks. Physical examination at presentation is remarkable only for the cutaneous manifestations of severe thrombocytopenia with bruising or a petechial rash present in almost all cases ( Table 1 ). Clinically significant lymphadenopathy or marked hepatosplenomegaly are atypical features; however, shotty cervical adenopathy is common in young children and a spleen tip may be palpable in 5% to 10% of cases. Epistaxis (often minor, sometimes severe) is a presenting symptom in approximately one quarter of affected children; hematuria occurs less frequently.
| Hemorrhagic Manifestations | ||||||
|---|---|---|---|---|---|---|
| Investigator | Number of Cases | Male:Female Ratio | Preceding Infectious Illness | Purpura/Petechiae | Epistaxis | Hematuria |
| Choi (1950–1964) a , | 239 | 117:122 | 119/239 | 235/239 | 76/239 | 20/239 |
| Lusher (1956–1964) | 152 | 69:83 | 122/146 | — | 46/152 | 8/152 |
| Blanchette (1974–1982) | 80 | 37:43 | 58/80 | 75/80 | 20/80 | 3/80 |
| Bolton-Maggs (1995–1996) | 427 | 213:214 | 245/427 | 310/427 | 85/427 | 6/427 |
| Total | 898 | 436:462 | 544/892 (60.9%) | 620/746 (83.1%) | 227/898 (25.3%) | 37/898 (4.1%) |
The key laboratory finding in children who have acute ITP is isolated, and often severe, thrombocytopenia. In more than half of cases, platelet counts at presentation are less than 20 × 10 9 /L ( Fig. 3 ). Other hematologic abnormalities are consistent with a diagnosis of childhood acute ITP only if they can be explained easily (eg, anemia secondary to epistaxis/menorrhagia) or atypical lymphocytosis in cases of infectious mononucleosis. The one exception is mild eosinophilia, which is a common finding. The blood smear shows a marked decrease in platelets with some platelets that are large (megathrombocytes) ( Fig. 4 ). A bone marrow aspirate, if performed, typically shows normal to increased numbers of megakaryocytes, many of which are immature (see Fig. 4 ). An increase in the number of bone marrow eosinophil precursors is present in some cases.
Natural History of Childhood Acute Immune Thrombocytopenic Purpura
The natural history of childhood acute ITP is well documented (reviewed by Blanchette and Carcao ). Complete remission, defined as a platelet count greater than 150 × 10 9 /L within 6 months of initial diagnosis and without the need for ongoing platelet-enhancing therapy, occurs in at least two thirds of cases. This excellent outcome seems independent of any management strategy. As an example, in the prospective study reported by Kühne and colleagues, complete remission rates of 68%, 73%, and 66% were reported in children who received no treatment, IVIG, or corticosteroids, respectively. These data are similar to the 76% complete remission rate reported by George and colleagues on the basis of a review of 12 case series involving 1597 cases. A recent study of children from five Nordic studies described a simple clinical score that predicts early remission. If confirmed, this could identify those children who might be left without active therapy for low platelet counts. Predictors of early remission were abrupt onset of illness, preceding infection, male gender, age under 10 years, wet purpura, and a platelet count less than 5 × 10 9 /L.
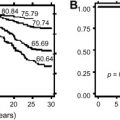
The outcome for children who have acute ITP who continue to manifest thrombocytopenia beyond 6 months from initial presentation generally is good. Published reports suggest that as many as one third of such children have spontaneous remission of their illness from a few months to several years after initial diagnosis. In one study, 61% was predicted at 15 years of follow-up. Most spontaneous remissions occur early, and the number of children who have severe thrombocytopenia (platelet counts <20 × 10 9 /L) and who are symptomatic with bleeding symptoms and, therefore, are therapy dependent more than 1 year after initial diagnosis is small. In a Swiss-Canadian retrospective analysis of 554 children who had newly diagnosed ITP and platelet counts less than 20 × 10 9 /L, the percentages of children who had platelet counts less than 20 × 10 9 /L at 6, 12, 18, and 24 months after diagnosis were 9%, 6%, 4%, and 3%, respectively ( Fig. 5 ). This is the small subgroup of children for whom splenectomy ultimately may need to be considered.
The case for treatment of children who have acute ITP relates to those who have significant bleeding and consideration of the very small, but finite, risk for intracranial hemorrhage (ICH). The risk of this feared complication was 0.9% in a series of 1693 children reviewed by George and colleagues. This figure, however, probably is an overestimate reflecting that reports in the literature mainly are from academic centers that likely are referred the most severe cases. Based on data in the United Kingdom, Lilleyman has estimated an incidence of 0.2% of ICH in children who have newly diagnosed ITP, a figure consistent with the 0.17% incidence rate (3 of 1742 children who had newly diagnosed acute ITP) reported by Kühne and colleagues on behalf of the ICIS.
Whatever the true incidence of ICH in children who have acute ITP, there is no doubt that this event is a devastating and sometimes fatal complication in this generally benign childhood disorder. The percent of cases of ICH occurring within 4 weeks of initial diagnosis varied from 19% to 50% in different reports; in one retrospective review, 10% (7/69) of cases of ICH occurred within 3 days of diagnosis of ITP. Trauma to the head and use of antiplatelet drugs, such as aspirin, were identified as risk factors for ICH in children who had ITP and very low platelet counts.
Unfortunately, a prospective randomized controlled trial to determine definitively whether or not therapeutic intervention can decrease the incidence of ICH significantly in children who have newly diagnosed ITP and platelet counts below 20 × 10 9 /L is not feasible, because of the large numbers of cases required to ensure a statistically significant outcome. Physicians who care for children who have acute ITP, therefore, must act in the best interest of each child without the benefit of definitive data. Because of the significant morbidity and mortality associated with ICH and the availability of highly effective platelet-enhancing therapies, some recommend that families of young children who have newly diagnosed acute ITP at risk for ICH (who have platelet counts <10 × 10 9 /L) be offered the option of treatment using the minimum therapy necessary to increase the platelet count rapidly to a safe, hemostatic level. There is no current evidence, however, that such a management strategy significantly reduces the incidence of ICH in children who have ITP, although intuitively this seems probable.
In addition, there is evidence to suggest that the rate of platelet response to frontline therapies (corticosteroids or IVIG) in the subset of children who have ITP and clinically significant hemorrhage is suboptimal. Discussion with parents and children, if of appropriate age, should include consideration of best available evidence with regard to the three key issues: (1) to treat or not to treat (2) to perform a bone marrow aspirate or not and (3) to hospitalize or not.
To treat or not to treat
Observation
The case for observation of children who have acute ITP rests with the knowledge that acute ITP is, for the majority of affected children, a benign self-limiting disorder, usually with mild clinical symptoms and has a low risk for serious bleeding (approximately 3% with ICH being rare) and the fact that there are no prospective studies that clearly indicate a decrease in the incidence of ICH associated with treatment. Several children who had ITP-associated ICH were receiving platelet-enhancing therapy at the time of the hemorrhage. In addition, all treatments suffer from the disadvantage of side effects, which can be severe.
Guidelines for initial management of children who have acute ITP have been published and reflect the ongoing debate, “to treat or not to treat”. Recommendations from the Working Party of the British Committee for Standards in Haematology General Haematology Task Force state that treatment of children who have acute ITP should be decided on the basis of clinical symptoms in addition to cutaneous signs, not the platelet count alone. The Working Party considered it appropriate to manage children who have acute ITP and mild clinical disease expectantly, with supportive advice, and a 24-hour contact point irrespective of the platelet count. Based on these guidelines, intervention is reserved for the few children who have overt hemorrhage and platelet counts below 20 × 10 9 /L or those who have organ- or life-threatening bleeding irrespective of the circulating platelet count. Many clinicians in Europe manage children who have ITP expectantly (ie, without medication to increase the platelet count) because of the rapid remissions in most cases, the low risk for bleeding, and toxicities of currently available medical therapies. Data are reported from the United Kingdom and Germany promoting the use of advice and support to children and their families during the usually short duration of the illness.
Corticosteroids
The corticosteroid treatment regimen used to treat children who have newly diagnosed ITP in most reported studies, and worldwide, is oral prednisone at a dose of 1 to 2 mg/kg per day given in divided doses and continued for a few weeks. Two randomized studies support the benefit of corticosteroid therapy in children who have ITP. In the first study, conducted by Sartorius and reported in 1984, 73 children ages 10 months to 14 years who had newly diagnosed ITP were randomized to receive oral prednisolone (60 mg/m 2 per day for 21 days) or a placebo. Platelet responses were significantly faster in the corticosteroid-treated group, with 90% of children achieving a platelet count of 30 × 10 9 /L within the first 10 days of treatment compared with 45% of children in the placebo no-treatment group. The Rumpel-Leede test, which measures capillary resistance (blood vessel integrity), became negative sooner in the corticosteroid-treated group. In the second study, reported by Buchanan and Holtkamp in 1984, 27 children who had acute ITP were randomized to receive oral prednisone (2 mg/kg per day for 14 days, with tapering and discontinuation of corticosteroids by day 21) or placebo. Although there was a definite trend in favor of corticosteroids, only on day 7 of therapy did the prednisone-treated patients have significantly higher platelet counts, lower bleeding scores, and shorter bleeding times than children receiving placebo. Taken together, these two studies suggest limited early benefit from conventional dose oral corticosteroid therapy in children who have acute ITP.
The risks and benefits of high-dose corticosteroid therapy administered orally or IV to children who have acute ITP merit discussion. In a study of 20 children randomized to receive oral megadose methylprednisolone (30 mg/kg for 3 days followed by 20 mg/kg for 4 days) or IVIG (0.4 g/kg × 5 days), Özsoylu and colleagues reported that 80% of children in both groups had platelet counts greater than 50 × 10 9 /L by 72 hours after the start of treatment. Corticosteroids were given before 9:00 am and adverse effects were not observed. In contrast, Suarez and colleagues reported that hyperactivity and behavioral problems occurred in 5 of 9 children who had acute ITP given 6 to 8 mg/kg per day of oral prednisone for 3 days or until platelet counts had increased to 20 × 10 9 /L. Immediate platelet responses with this regimen were impressive: the mean time to achieve a platelet count of 20 × 10 9 /L was 1.9 ± 0.6 days (range 1–3 days).
A commonly used high-dose corticosteroid regimen is that reported by van Hoff and Ritchey. The investigators treated 21 consecutive children who had ITP using IV methylprednisolone (30 mg/kg, maximum dose 1 g) given daily for 3 days. The median time to achieving a platelet count greater than 20 × 10 9 /L was 24 hours. Ten children (48%) had transient glycosuria but no cases of hyperglycemia were observed. Similar results were reported by Jayabose and colleagues, who treated 20 children who had acute ITP with IV methylprednisolone (5 mg/kg per day in four divided doses). By 48 hours from start of treatment, 90% of children had platelet counts greater than 20 × 10 9 /L, and all children achieved this hemostatic threshold by 72 hours from the start of treatment. No patients developed symptomatic hyperglycemia or hypertension; the investigators did not comment about weight gain or mood/behavioral changes. The authors’ experience with short-course oral prednisone (4 mg/kg per day × 4 days without tapering) is complementary. Eighty-three percent of children who had acute ITP and platelet counts less than 20 × 10 9 /L achieved a platelet count above 20 × 10 9 /L within 48 hours of starting corticosteroid therapy ( Fig. 6 ).