Type
Prevalence/histology
Genetic alterations
Pertinent molecular pathways
Papillary RCC type 1 and 2
10–15 %
Type 1 with small cells and little cytoplasm; basophilic
Type 2 with large cells and eosinophilic cytoplasm; hypovascular
Trisomy of chromosome 7 and 17/del Y in type 1
Multiple cytogenetic abnormalities in type 2
Dysregulation of G1-S checkpoint genes and G2-M checkpoint genes
c-MET proto-oncogene-activating mutation leading to constitutive activation of the hepatocyte growth factor (HGF)/MET pathway
VEGF and VEGFR expression
Chromophobe RCC
4–10 %
Large polygonal cells with transparent or reticulated cytoplasm
Eosinophilic variant with purely eosinophilic cells
LOH at multiple chromosomes
Hypodiploid DNA content
VEGF and upregulation of c-KIT has been noted in tumor specimens
In familial chromophobe RCC, a tumor suppressor, folliculin, has been identified and may be associated with the mTOR pathway
Collecting duct RCC (Bellini duct)
0.5–2 %
Irregularly angulated glands
Desmoplastic stroma
Monosomies
LOH of 8p,
13q, 1q, 6p, 9p, 21q
High incidence of c-ErbB-2 oncogene amplification
Renal medullary carcinoma
Less than 1 %
Poorly differentiated with rhabdoid elements
Eosinophilic with clear nuclei
Rare 22q11.2 inactivation (INI1/hSNF5 tumor suppressor)
BCR and ABL gene amplification without BCR-ABL translocation
Topo II overexpression
Xp11 translocation carcinoma
Less than 2 %
Both clear cells and papillary architecture
Psammoma bodies
Various translocations of Xp11.2
Translocations of chromosome Xp11.2 leading to gene fusions of transcription factor E3
Table 21.2
Targeted treatment options for NCCRCC
Type | Study | N/agent | Relevant outcomes |
|---|---|---|---|
NCCRCC not further subclassified or multiple subtypes included in study | Rowinsky et al. (2004) [94] | 14/panitumumab | 14 % PR; 43 % SD; PFS of 92 days |
Ronnen et al. (2006) [93] | ~12/bortezomib | 1 CR in a RMC patient | |
Dutcher et al. (2009) [29] | ~120/temsirolimus | OS 11.6 months; PRCC in ~75 %; CHRCC in ~10–15 % | |
Gore et al. (2009) [39] | 437/sunitinib | ORR 11 %; 57 % SD | |
Plimack et al. (2009) [85] | 6/BRYO | At least 1 PR seen | |
Molina et al. (2012) [72] | 22/sunitinib | 5 % PR; 71 % SD; PFS 5.5 months | |
Grünwald et al. (2012) [40] | 75/everolimus | Median treatment duration 12 weeks; 1.3 % PR rate | |
Koh et al. (2013) [50] | 49/everolimus | PR in 10.2 %, PFS 5.2 months | |
Matrana et al. (2014) [64] | 29/pazopanib | PFS/OS was 8.1/31.0 months for frontline use with 33 % RR; PFS/OS was 4.0/13.6 months in salvage setting with 6 % | |
Voss et al. (2014) [124] | 85/everolimus or temsirolimus | RR 7 %; SD 49 %; PFS/OS was 2.9 and 8.7 months | |
Papillary RCC | Ratain et al. (2006) [88] | 15/sorafenib | 13 % PR |
Ronnen et al. (2006) [93] | 3/sunitinib | 33 % PR; PFS 8.5 months | |
Choueiri et al. (2008) [15] | 41/sorafenib or sunitinib | 4.8 % PR; PFS of 7.6 months overall and 11.9 months with sunitinib | |
Srinivasan et al. (2008) [103] | 25/GSK1363089 | 16 % PR; 80 % SD | |
Gordon et al. (2009) [38] | 45/erlotinib | 11 % PR; OS 27 months | |
Ravaud et al. (2009) [89] | 28/sunitinib | 4 % PR; 57 % SD | |
Stadler et al. (2010) [105] | 107/sorafenib | 3 % PR; SD 81 % | |
Beck et al. (2011) [9] | 112/sorafenib | ~4 % PR;~6.6 month PFS | |
Lee et al. (2012) [56] | 31/sunitinib | 22 patients with PRCC with ORR of 36 %; overall study PFS 6.4 months | |
Tannir et al. (2012) [114] | 55/sunitinib | 27 patients with PRCC, PFS 1.6 months, OS 12.6 months; 0 % ORR | |
Choueiri et al. (2013) [16] | Foretinib | 13.5 % RR; PFS 9.3 months | |
Escudier et al. (2013) [32] | 92/everolimus | PFS 3.7 months; OS 21 months | |
Stamatakis et al. (2013) [109] | 34/erlotinib with bevacizumab | In sporadic cases, 25 % PR; 42.9 % PR in HLRCC | |
Tannir et al. (2014) [113] | 68/Sutent vs. everolimus with a crossover upon progression | 27 patients with PRCC, OS 14.9 months for everolimus, 16.6 for sunitinib, 0 % ORR | |
Yildiz et al. (2014) [130] | 63/sunitinib | 46 patients with PRCC; overall RR 11.1 %, PFS 7.6 months | |
Chromophobe RCC | Choueiri et al. (2008) [15] | 12/sorafenib or sunitinib | 25 % PR; 75 % SD; PFS 10.6 months |
Stadler et al. (2010) [105] | 20/sorafenib | 5 % PR | |
Collecting duct RCC (Bellini Duct) | Tannir et al. (2011) [112] | 20/sunitinib or bevacizumab or other | Median OS of 421 days; one PR |
Pecuchet et al. (2013) [81] | Bevacizumab/gemcitabine/platinum | 3 PRs, 1 SD, and 1 CR after a metastasectomy; mPFS 15.1 months and mOS 27.8 months | |
Xp11 translocation carcinoma | Malouf et al. (2010) [61] | 11/sunitinib; 1/temsirolimus | PFS 8.2 months; 9 % CR, 27 % PR; 55 % SD all with sunitinib |
RCC with sarcomatoid dedifferentiation | Golshayan et al. (2009) [37] | 43/sunitinib or sorafenib or bevacizumab | 19 % PR; 49 % SD; PFS 5.3 months; OS 11.8 months |
Beck et al. (2011) [9] | 53/sorafenib | PFS of 4 months |
21.2 Histopathologic, Genetic, and Molecular Considerations of Non-clear Cell Renal Cell Carcinoma
Detailed pathologic and molecular biologic characteristics of RCC generally and non-clear subtypes specifically are discussed in Chaps. 1 and 2. There are now 14 NCCRCC subtypes identified [59, 102]. Briefly, the major non-clear subtypes include [59]:
21.2.1 Papillary Carcinoma (PRCC)
Papillary carcinoma (PRCC) is thought to arise from either the proximal or distal convoluted tubules of the nephron and accounts for 10–15 % of localized RCC in most large series [13, 71, 80]. In approximately 10 % of PRCC cases, tumors are multifocal or bilateral [101]. Two morphologic subtypes of PRCC, type 1 with small cells and little cytoplasm and type 2 with large cells and eosinophilic cytoplasm, have been identified and shown to have different genetic profiles (Fig. 21.1a, b) [2, 25, 52, 71, 90]. Further work has suggested two separate molecular classes of PRCC: the first class, exhibiting excellent survival, has dysregulation of G1-S checkpoint genes and higher c-MET expression and combines morphologic type 1, low-grade type 2, and mixed type 1/low-grade type 2 tumors, and the second class, exhibiting poor survival, has dysregulation of G2-M checkpoint genes and is morphologically composed of high-grade type 2 tumors [129]. Although hereditary PRCC is associated with activating MET mutations [96, 100], histologically this tumor appears to be most similar to sporadic type 1 PRCC, and only about 14 % of patients with sporadic PRCC harbor a MET mutation [60, 97]. PRCC can also be seen in the hereditary leiomyomatosis and RCC syndrome due to fumarate hydratase (FH) tumor suppressor inactivation, and this appears to be histologically most similar to type 1 PRCC; FH mutations have not been described in sporadic cases [48, 55, 119]. Finally, vascular endothelial growth factor (VEGF) and VEGF receptors (VEGFR) have been shown to also be expressed in PRCC, but the clinical correlation remains unclear [28, 58]. Tubulocystic renal cell carcinoma and mucinous, tubular, and spindle cell carcinoma are two newer renal cancer subtypes that have been proposed to be additional variants of PRCC [59, 102].
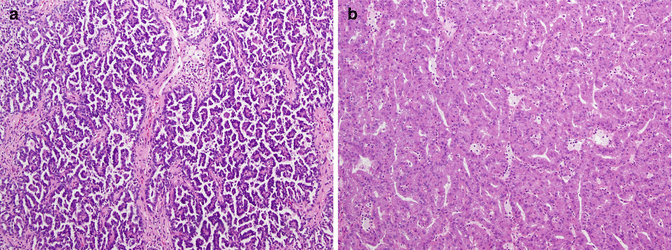

Fig. 21.1
(a) Papillary renal cell carcinoma type 1. The papillary cores with foamy macrophages are lined by small cuboidal cells with low-grade nuclei and minimal amount of cytoplasm. (b) Papillary renal cell carcinoma type 2. In this type of tumor, the papillary cores are lined by cells with abundant acidophilic cytoplasm and typically have high-grade nuclei with prominent nucleoli (Courtesy of Dr. Tatjana Antic, University of Chicago, Department of Pathology)
21.2.2 Chromophobe RCC (CHRCC)
Chromophobe RCC (CHRCC) is thought to originate from the intercalated cells in the renal collecting ducts and accounts for approximately 4–10 % of RCC (Fig. 21.2) [4, 9, 19]. In familial chromophobe RCC associated with Birt-Hogg-Dubé (BHD) syndrome, inactivation of a tumor suppressor, folliculin, has been identified and may activate the mTOR pathway, but folliculin alterations have rarely been found in sporadic CHRCC [43, 75, 123]. VEGF and upregulation of c-KIT have been noted in tumor specimens, although activating mutations of c-KIT have not been found [28, 95, 110, 127].
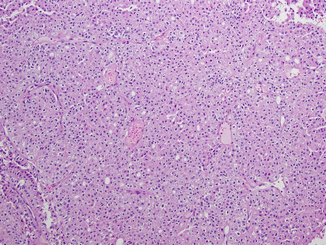

Fig. 21.2
Chromophobe renal cell carcinoma. The tumor cells are arranged in nests divided by interspersed thin-walled blood vessels. The cells contain eosinophilic cytoplasm with prominent cell membranes and dark resinoid nuclei with perinuclear halos (Courtesy of Dr. Tatjana Antic, University of Chicago, Department of Pathology)
21.2.3 Collecting Duct RCC (CDRCC)
Collecting duct RCC (CDRCC) likely arises from the collecting (Bellini) ducts of the kidney in the renal medulla and is an aggressive RCC subtype with approximately one third of patients presenting with metastatic disease (Fig. 21.3) [69]. A relationship to urothelial cell carcinoma has been proposed [125].
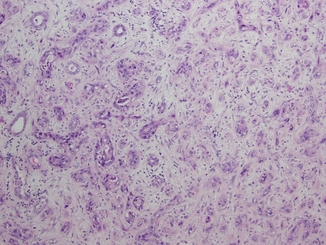

Fig. 21.3
Collecting duct carcinoma. Malignant cells with high-grade nuclear features arranged in tubules and cords are infiltrating the renal medulla. Pronounced desmoplastic stromal reaction is present (Courtesy of Dr. Tatjana Antic, University of Chicago, Department of Pathology)
21.2.4 Renal Medullary Carcinoma (RMC)
Renal medullary carcinoma (RMC), a rare, aggressive, and usually fatal RCC variant, is a close relative of CDRCC (Fig. 21.4) [22, 128]. Almost all RMC occurs in children and young adults with sickle cell trait or disease.
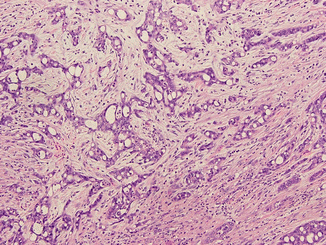

Fig. 21.4
Medullary renal cell carcinoma. The tumor is composed of fusing tubules and cords made of pleomorphic malignant cells in desmoplastic stroma. The acute inflammatory infiltrate is commonly seen in this type of tumor (Courtesy of Dr. Tatjana Antic, University of Chicago, Department of Pathology)
21.2.5 MiT Family Translocation RCC
MiT family translocation RCC involves different translocations of the TFE3, TFEB, TFEC, and MiTF transcription factors typically involving chromosome Xp11.2 but also including (6:11) translocations [6, 10, 102]. This was previously thought to be an extremely rare entity seen exclusively in children and young adults, but large series showed that 15 % of RCC patients under the age of 45 had this subtype of RCC. Although certain specific translocations can have indolent behavior, the majority of cases seen in adults are very aggressive [51].
21.2.6 Sarcomatoid Dedifferentiation
Sarcomatoid dedifferentiation, first described by Fallow et al. in 1968, is not a separate histologic subtype but rather a variant that is observed with any RCC histology, can involve 1–100 % of a given tumor, and is seen in 5–10 % of RCC based on a number of large surgical series; although, it has been described to occur up to 30 % in CDRCC [12, 23, 35]. It exhibits a spindle cell pattern of growth, is always a high-grade tumor, and has been associated with the expression of VEGF, c-Kit, PDGFR-alpha, and S6 kinase as well as p53 mutations and is associated with poor prognosis (Fig. 21.5) [24, 78, 83, 117].
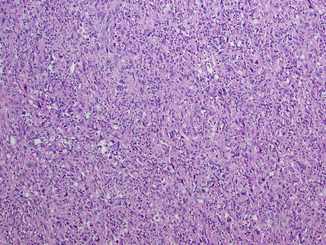

Fig. 21.5
Sarcomatoid dedifferentiation. The sarcomatoid change can be seen in any type of renal cell carcinoma with tumor showing highly pleomorphic cells in storiform pattern, numerous mitotic figures, and apoptotic bodies, simulating sarcoma-like appearance (Courtesy of Dr. Tatjana Antic, University of Chicago, Department of Pathology)
21.3 Survival Considerations: Non-clear Cell Renal Cell Carcinoma
Because optimal therapies for NCCRCC remain unknown, available therapies can have significant toxicities, and certain subtypes have an indolent natural history; knowledge of expected survival in the absence of treatment is critical to therapeutic decision-making. Most studies evaluated outcome in surgical series of primary nephrectomies, but with those caveats, we will review the salient findings.
Localized PRCC and CHRCC have in some studies, but not others, been shown to have an improved survival compared to localized CCRCC. In a large multicenter retrospective series of 4,063 patients for those with localized disease, 5-year survival rates were 73.2 %, 79.4 %, and 87.9 % for clear cell, papillary, and chromophobe carcinoma, respectively, but once adjusted for TNM stage, no significant survival difference was observed [80]. A study of 2,385 patients treated at the Mayo Clinic from 1970 to 2000 found the 5-year cancer-specific survival for the entire group to be 68.9 % in those with CCRCC, 87.4 % in PRCC, and 86.7 % in CHRCC patients with CCRCC patients having a statistically worse outcome even after stratifying for tumor stage and nuclear grade (P < 0.001) [13]. CHRCC has a low rate of metastasis (~5 %) with a 5-year OS of 92 % seen in a series of 50 patients and has been shown to have a statistically significant less chance of disease recurrence compared to CCRCC after a nephrectomy [4, 8, 19, 116].
Metastatic PRCC and CHRCC appear in most studies to have a worse prognosis compared to CCRCC. A study of 64 patients with metastatic non-clear cell RCC treated with both cytokine and conventional chemotherapy agents found that only two had a partial response with a median OS of 9.4 months with 29 months for those with CHRCC, 11 months for those with CDRCC, and 5.5 months for those with PRCC [73]. These same investigators reviewed 353 previously untreated metastatic RCC, of whom 13 % had NCCRCC (mostly papillary), and those with CCRCC had a significantly better survival than those of NCCRCC [66]. Another series of 38 patients with metastatic PRCC had an OS of 8 months, and a separate single-center study found a significant difference in survival of patients with metastatic RCC after a cytoreductive nephrectomy with 9.1-month median OS in those with PRCC vs. 22 months for CCRCC [63, 93]. A retrospective analysis of the International mRCC Database Consortium (IMDC) included 252 patients with NCCRCC of whom 151 had PRCC and 37 had CHRCC [53]. The median OS for the NCCRCC subgroup was 12.8 months compared to 22.3 months in the clear-cell RCC group. Interestingly, the median OS for PRCC was 14.0 months (adjusted hazard ratio for death 1.57 compared to CCRCC, p < 0.0001) versus 27.1 months for those with CHRCC (adjusted hazard ratio for death 0.89 compared to CCRCC, p = 0.646).
CDRCC and RMC patients have in general uniformly poor survival even when localized. A study of 160 cases noted the 3-year disease-specific survival was 58 % compared to 79 % for CCRCC and for those with metastatic disease a median OS of 5 months for CDRCC versus 8 months in those with CCRCC [125]. In another study of 26 patients with metastatic CDRCC, the median OS was 11 months with a 5 % 2-year survival [73]. CDRCC OS at 5 and 10 years for a cohort of 81 patients in Japan was 34.3 % and 13.7 %, respectively, with 32 % presenting with metastatic disease [118]. For RMC, mean survival in several series has been approximately 4 months [111].
Sarcomatoid dedifferentiation has clearly been demonstrated to be a poor prognostic marker. In a large series of 2,381 patients, 120 (5 %) of whom had a sarcomatoid component in various stages of RCC, the 5-year cancer-specific survival was 14.5 %, and the presence of a sarcomatoid component was significantly associated with death [12]. Sarcomatoid dedifferentiation has also been shown to be an independent poor prognostic marker in metastatic RCC in those treated with cytokine therapy with one study showing a median OS to be 22 months vs. 10 months in those treated with cytokines and having no sarcomatoid vs. sarcomatoid features [54, 57, 62].
In summary, when localized, CCRCC appears to have a worse prognosis than PRCC or CHRCC, but this is not necessarily true in the metastatic setting, including under treatment with cytokines or conventional chemotherapy, which have minimal to no efficacy. CDRCC/RMC and sarcomatoid dedifferentiation lead to abysmal outcomes. Outcomes with conventional chemotherapy, cytokines, VEGF pathway, and mTOR-targeted therapies for NCCRCC are discussed below.
21.4 DNA and DNA Repair-Targeted Therapy of Non-clear Cell Carcinoma
Conventional cytotoxic chemotherapy has not been considered to be useful in the treatment of RCC; nevertheless, objective responses have been reported using nucleoside analog-based therapies with the highest response rates reported utilizing a combination of gemcitabine and a fluoropyrimidine (capecitabine most often) or gemcitabine with doxorubicin [18, 68, 104, 106, 115, 126]. Application of these and other cytotoxic therapies in the NCCRCC specifically is summarized below [21, 44].
21.4.1 PRCC
PRCC is in general resistant to conventional chemotherapy [17]. A retrospective study of 38 patients with metastatic PRCC, 30 of whom were treated with systemic therapy of which six received conventional chemotherapy, showed no objective responses [93]. In a study of 153 patients treated with gemcitabine and 5-fluorouracil, two had definite PRCC and neither showed an objective response [107]. A phase I study of gemcitabine, capecitabine, IFN-α, and thalidomide in 12 patients included two with PRCC, one of whom had a partial response [3]. An analysis of 18 patients with PRCC treated with various agents including conventional chemotherapy showed no significant responses [73]. A phase II study of 45 patients with mRCC receiving S-1 (an oral formulation combining tegafur, 5-chloro-2,4-dihydropxypyridine, and potassium oxonate) included eight patients with PRCC; ORR was 12.5 % and PFS 5.9 months [76]. Another phase II study of carboplatin and paclitaxel for 16 PRCC patients showed no responses [11]. Finally, a phase II study of single-agent capecitabine in previously untreated metastatic NCCRCC patients enrolled 51 individuals (39 PRCC, 7 CHRCC, 5 CDRCC) most of whom had an intermediate MSKCC risk score and all of whom had a prior nephrectomy and surprisingly high response rates of 2 complete responses (CR), 11 partial responses (PR), and 24 with stable disease (SD) with a median PFS, and OS of 10.1 months and 18.3 months, respectively, was reported [120]. One of the CRs occurred in a PRCC patient.
21.4.2 CHRCC
CHRCC has rarely been evaluated in regard to conventional chemotherapy use, but in 12 individuals in a series of 64 patients with metastatic NCCRCC, none had a response to conventional chemotherapy, although specific agents used were not specified [73]. One CR out of seven CHRCC patients was observed in a series of 51 NCRCC patients treated with single-agent capecitabine [120]. No responses were seen in two patients with CHRCC receiving pemetrexed plus gemcitabine [91].
21.4.3 CDRCC and RMC
CDRCC and RMC treatment with conventional chemotherapy has been evaluated in a number of retrospective series and case reports due to histologic similarities between CDRCC and urothelial carcinoma. In a series of 64 patients with NCCRCC, 26 had collecting duct/medullary histology, and one had a 5-month PR to gemcitabine plus cisplatin therapy [73]. A series of 12 patients with CDRCC treated most commonly with methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) reported only one response lasting 5 months [27]. The largest retrospective series of CDRCC included 81 patients from Japan and included 26 patients with distant metastatic disease. Almost everyone was initially treated surgically, and 17 individuals were treated postoperatively with chemotherapy with only a single response to combination of gemcitabine and carboplatin [118]. In a separate study of nine patients with CDRCC treated with gemcitabine and cisplatin, two CRs were noted [84]. This was followed by a prospective phase II trial of gemcitabine and cisplatin or carboplatin for 23 treatment-naive metastatic CDRCC patients from six French centers with an objective response (CR + PR by Response Evaluation Criteria in Solid Tumors) rate of 26 % (one CR and five PR) and a median PFS and OS of 7.1 months and 10.5 months, respectively [79]. The previously mentioned small phase II trial of pemetrexed and gemcitabine had no activity in three CDRCC patients and in NCCRCC in general [91]. Finally, a series of five patients treated with gemcitabine, platinum, and bevacizumab with bevacizumab maintenance led to three cases of PR, one case of SD, and one CR after a metastasectomy; mPFS was 15.1 months and mOS 27.8 months [81].
21.4.4 Sarcomatoid Dedifferentiation
Sarcomatoid dedifferentiation leads to an aggressive growth pattern and uniformly poor prognosis. Early studies of MAID (mesna, adriamycin, ifosfamide, dacarbazine), gemcitabine/docetaxel/carboplatin, and doxorubicin-based CYVADIC treatments showed limited success [7, 20, 45, 98]. A phase II trial of doxorubicin and ifosfamide in 25 patients with metastatic sarcomatoid RCC showed no objective response and a median OS of 3.9 months, although case reports of CRs to the same chemotherapy can be found [33, 87]. Eighteen patients with sarcomatoid features treated with a combination of doxorubicin and gemcitabine had two CRs, five PRs, and one SD (ORR = 39 %), and long-term follow-up of four patients found the two complete responders alive 6 and 8 years later and the other two surviving over 3 and 6 years [30, 77]. An ECOG phase II trial of doxorubicin and gemcitabine in 39 patients with sarcomatoid features showed a 16 % response rate (five PRs and one CR) and 10 (26 %) with SD with a median OS of 8.8 months and a median PFS of 3.5 months [41]. It also appeared that those tumors with a higher sarcomatoid component responded better to the chemotherapy. Of note, these trials made no attempt to distinguish the histologic origin or specific renal cancer subtype. A trial of sunitinib with or without gemcitabine for mRCC patients with sarcomatoid features is ongoing (clinicaltrials.gov NCT01164228).
In summary, nucleoside analog therapy appears to have similar low level activity in PRCC and CHRCC as it does in CCRCC, with the caveat that the clinical significance is unknown. CDRCC occasionally responds to agents typically utilized for urothelial cancer, but complete responses are extremely rare, and response rates and response durations appear to be less than in typical urothelial cancer. Reports of complete responses with gemcitabine and doxorubicin in RCC with sarcomatoid differentiation remain intriguing but have not been uniformly replicated, and it has not been possible to determine whether such responses are limited to any specific RCC subtype.
21.5 Cytokine Therapy of Non-clear Cell Renal Cell Carcinoma
Although cytokine therapy has been useful in CCRCC with responses seen in up to 20 % of patients with complete responses in 5–10 % of patients treated with high-dose IL-2, it has not been successful in NCCRCC [18]. In an analysis of 163 cases of metastatic RCC treated with IL-2 for whom kidney specimens were available and who were treated between 1990 and 2001, 146 were CCRCC and 17 NCCRCC with two PRCC type 1, nine PRCC type 2, two chromophobe, and one CDRCC. Response rate (>50 % regression of measurable tumor) to IL-2 was observed in 30 of the CCRCC patients (21 %) versus one of the NCCRCC patients (6 %) which was reportedly a CDRCC case [121]. Preliminary results of a prospective single-arm SELECT trial, with 120 patients treated with high-dose IL-2, showed an objective response in 0 out of five NCCRCC patients [65]. A review of 31 patients with NCCRCC treated with either IL-2, IFN-α, or the combination of the two revealed one PR in a patient with CHRCC treated with IFN-α [73]. Dimopoulos et al. treated six CDRCC patients with a combination of IL-2 and IFN-α with one PR and two SDs [27]. Tokuda et al. found no response to immunotherapy in 34 patients treated with CDRCC [118]. Finally, a study of 108 patients with sarcomatoid features, but no information on the underlying histology, of whom 80 received some form of immunotherapy either alone or in combination with conventional chemotherapy, revealed 28 PRs and no CRs, with an OS for the entire cohort of 9 months [67]. Thus, although cytokine therapy is useful in select cases of metastatic CCRCC with CR being occasionally observed, there is little evidence to support its use in those with NCCRCC.
21.6 VEGF Pathway-Targeted Therapy of Non-clear Cell Renal Cell Carcinoma
The VHL/HIF pathway that is abnormal in most patients with sporadic CCRCC is not thought to be a major driver in any NCCRCC; nevertheless, VEGF and VEGF receptors are present and apparently overexpressed, at least in PRCC and CHRCC [58]. Multiple VEGF pathway-directed agents have been approved for CCRCC based on a number of large phase III trials which showed improved PFS and trends toward OS benefit in the metastatic CCRCC setting [31, 34, 74, 92]. Not surprisingly, these agents have also been investigated in NCCRCC; although, there are limited prospective evaluations.
21.6.1 Papillary RCC
In the randomized discontinuation trial of sorafenib, 15 patients with PRCC were included and two attained a PR [88]. A retrospective analysis examining 41 metastatic PRCC patients treated with sorafenib or sunitinib between 2002 and 2006 at four European and one American center showed a PFS of 7.6 months, with a response rate of 4.8 % (two patients, both treated with sunitinib and achieving a PR) and a PFS of 11.9 vs. 5.1 months in those treated with sunitinib vs. sorafenib (P < 0.001) [15]. Two studies, the EU-ARCCS (Advanced Renal Cell Carcinoma Sorafenib) and the North American ARCCS, examined sorafenib use in a community-wide, expanded-access manner and analyzed its data on NCCRCC [8, 101]. The North American ARCCS trial evaluated 1,891 patients for RECIST response of whom 107 had PRCC. The CR, PR, SD, and CR+PR+SD rates for all patients and PRCC were CR = <1 %, 0 %; PR = 4 %, 3 %; SD = 80 %, 81; CR+PR+SD = 84 %, 84 %, respectively. The EU-ARCCS looked at 1,150 patients, with a 4 % PR rate and a PFS of 6.6 months; a subset analysis of those with papillary features (n = 112) found the PR and PFS to be similar to the clear-cell subset [8]. An expanded-access trial of sunitinib of over 4,300 patients, 68 % of whom had prior cytokine therapy and 3,464 of whom were evaluable, showed an overall objective response (CR +PR) of 17 % (1 % CR) and SD of >3 months in 59 % [39]. In the study, there were 437 (13 %) NCCRCC (not further subclassified) patients able to be evaluated, and an ORR of 11 % (2 CRs) and SD of 57 % was achieved, both comparable to the entire cohort. A phase II sunitinib trial enrolled 25 PRCC patients of whom 74 % had either poor or intermediate risk by MSKCC criteria and no PRs or CRs were seen, and median PFS and OS were only 1.6 and 12.6 months, respectively [110]. Another phase II trial of sunitinib in patients with NCCRCC included 22 evaluable patients, eight of whom had PRCC and achieved a PFS of 5.6 months with OS not reported [70]. A phase II trial of sunitinib in five patients with type 1 PRCC and 23 patients with type 2 PRCC showed a PR in one type 2 patient with 13 SD and 3/5 patients with SD in the type 2 [86]. A multicenter phase II trial from South Korea reported on 31 NCCRCC patients, of whom 22 had PRCC, treated with sunitinib; for the entire cohort, the median PFS was 6.4 months, and for the PRCC patients, the ORR was 36 % [55]. Another multicenter trial from the Turkish Oncology Group reported on 63 NCCRCC patients treated with sunitinib with 88 % having PRCC [126]. Overall study RR and disease control rate was 11.1 % and 63.5 %; median PFS and OS were 7.6 months and 22 months. Finally, two prospective trials of sunitinib versus everolimus in NCCRCC have been completed, and one has been reported in preliminary fashion [109]. This trial was aborted early due to improved overall survival in the sunitinib arm. Notably, 27 of 68 enrolled patients had PRCC, and their estimated median OS in the sunitinib and everolimus arms was 16.6 and 14.9 months, respectively; notably, no objective responses with first-line therapy in PRCC patients were seen. Differences in outcomes seen between the expanded-access trials and the phase II data may be related to lack of central pathologic review in the large trials as well as a selection bias and possibly ethnic differences in response to the phase II trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree